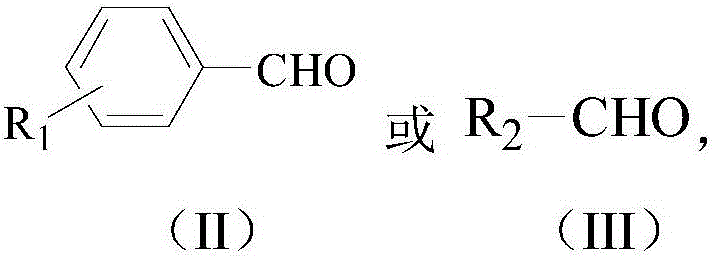
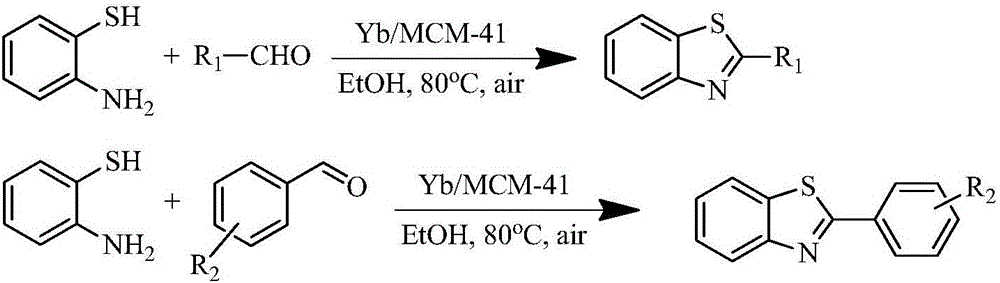
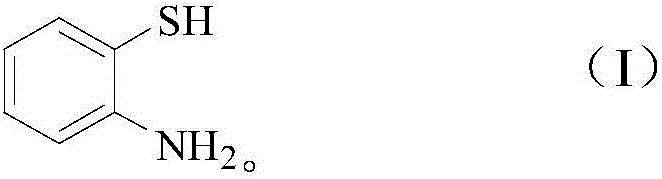Method for catalytic synthesis of 2-substituted benzothiazole compound by utilizing Yb/MCM-41 molecular sieve-based catalyst
A technology of MCM-41 and benzothiazole, which is applied in the field of rare earth Lewis acid-supported catalysts, can solve the problems of unfavorable catalyst separation, increased cost, and inability to recycle, and achieve high yield, favorable industrial production, and high loading rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1. Synthesis of Yb / MCM-41 molecular sieve catalyst
[0041] (1) Add cetyltrimethylammonium bromide (CATB), ethylamine (EA), deionized water, and magnetons into a round-bottomed flask, stir with a magnetic stirrer, and add orthosilane dropwise while stirring Ethyl Oxide (TEOS), solid dissolved and added dry YbCl 3 , where n(TEOS):n(CTAB):n(YbCl 3 ): n(H 2 O)=1:0.2:0.05:100;
[0042] (2) After stirring for 4 hours, the solids are all dissolved, take out the magnet and wash it, transfer the reactant to a polytetrafluoroethylene inner container, the filling amount of the inner container is 70%, put the inner container into a stainless steel shell, and tighten it The hydrothermal reaction kettle is ready for use;
[0043] (3) Put the hydrothermal reaction kettle into an oven and heat treat at 100°C for 48 hours;
[0044] (4) After the hydrothermal reaction is completed, the mixture is cooled and centrifuged, and the supernatant is evaporated and dried to obtain unreacte...
Embodiment 2
[0051] 1. Synthesis of Yb / MCM-41 molecular sieve catalyst
[0052] (1) Add cetyltrimethylammonium bromide (CATB), ethylamine (EA), deionized water, and magnetons into a round bottom flask, pH = 8-9, stir with a magnetic stirrer, and Add tetraethyl orthosilicate (TEOS) dropwise while stirring, add dry YbCl after the solid dissolves 3 , where n(TEOS):n(CTAB):n(YbCl 3 ): n(H 2 O)=1:0.1:0.07:80;
[0053] (2) After stirring for 4 hours, the solids are all dissolved, take out the magnet and wash it, transfer the reactant to a polytetrafluoroethylene inner container, the filling amount of the inner container is 60%, put the inner container into a stainless steel shell, and screw it tightly The hydrothermal reaction kettle is ready for use;
[0054] (3) Put the hydrothermal reaction kettle into an oven and heat treat at 90°C for 72h;
[0055] (4) Cool the mixture after the completion of the hydrothermal reaction and centrifuge at a speed of 3000-5000r / min, each time for 2-4min, r...
Embodiment 3
[0061] 1. Synthesis of Yb / MCM-41 molecular sieve catalyst
[0062] (1) Add cetyltrimethylammonium bromide (CATB), ethylamine (EA), deionized water, and magnetons into a round-bottomed flask, stir with a magnetic stirrer, and add orthosilane dropwise while stirring Ethyl Oxide (TEOS), solid dissolved and added dry YbCl 3 , where n(TEOS):n(CTAB):n(YbCl 3 ): n(H 2 O)=1:0.3:0.03:120;
[0063] (2) After stirring for 4 hours, the solids are all dissolved, take out the magnet and wash it, transfer the reactant to a polytetrafluoroethylene inner container, the filling amount of the inner container is 65%, put the inner container into a stainless steel shell, and tighten it The hydrothermal reaction kettle is ready for use;
[0064] (3) Put the hydrothermal reaction kettle into an oven and heat-treat at 110°C for 36 hours;
[0065] (4) Cool the mixture after the completion of the hydrothermal reaction and centrifuge at a speed of 3000-5000r / min, each time for 2-4min, repeat 3-4 ti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com