Diagnosis kit and method for detecting human c-kit gene exon 9 mutation
A kit and human technology, applied in the directions of biochemical equipment and methods, microbial determination/inspection, DNA/RNA fragments, etc., can solve the problem of inability to evaluate multiple mutation sites in exon 9, time-consuming, and detection of sites. Single point and other problems, to achieve the effect of good clinical application prospects, rapid detection, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 The present invention detects the kit and detection method of exon 9 mutation of C-Kit gene
[0035] One, the composition of kit of the present invention
[0036] PCR amplification reagent (1 serving):
[0037] water
14.85μl
10XPCR buffer
2.5μl
MgCl 2 (25mM)
0.75μl
UTP PLUS
0.5μl
Primer solution
1μl
UNG
0.2μl
Taq
0.2μl
overall system
20μl
[0038] Wherein "Primer solution" is prepared as follows:
[0039] C9F (100nM / ml)
3.125μl
C9F2 (100nM / ml)
3.125μl
C9R (100nM / ml)
3.125μl
C9P (100nM / ml)
0.625μl
H2O
15μl
total
25μl
[0040] The amplification primers and detection probes for mutations in exon 9 of the c-kit gene are as follows:
[0041]
[0042] External control primers and probes:
[0043]
[0044] 2. Using the kit of the present invention to detect mutations in exon 9 of the C-Kit ...
experiment example 1
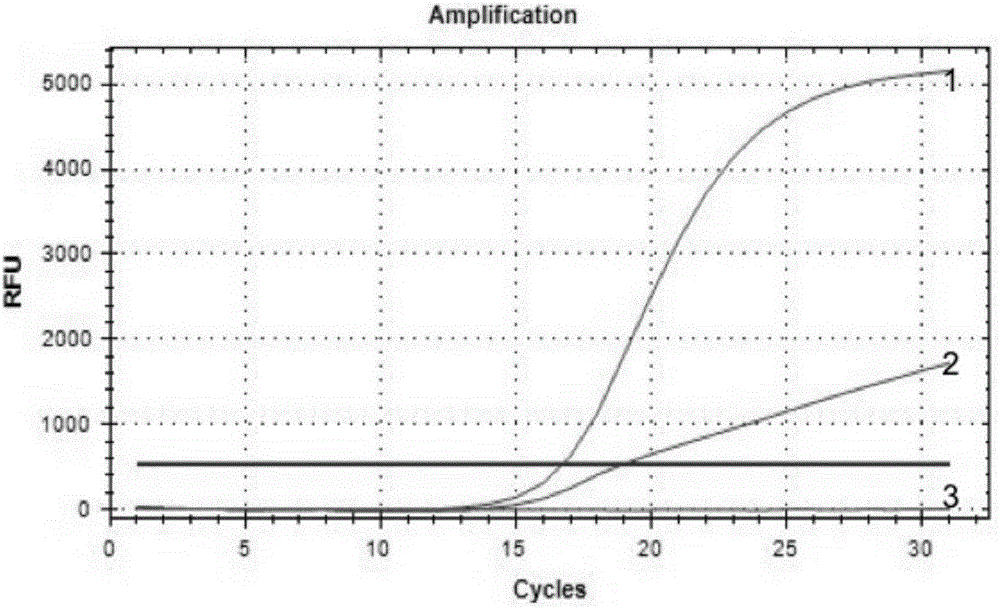
[0080] Experimental example 1 The accuracy and specificity detection of kit of the present invention and method
[0081] 1. Sample to be tested
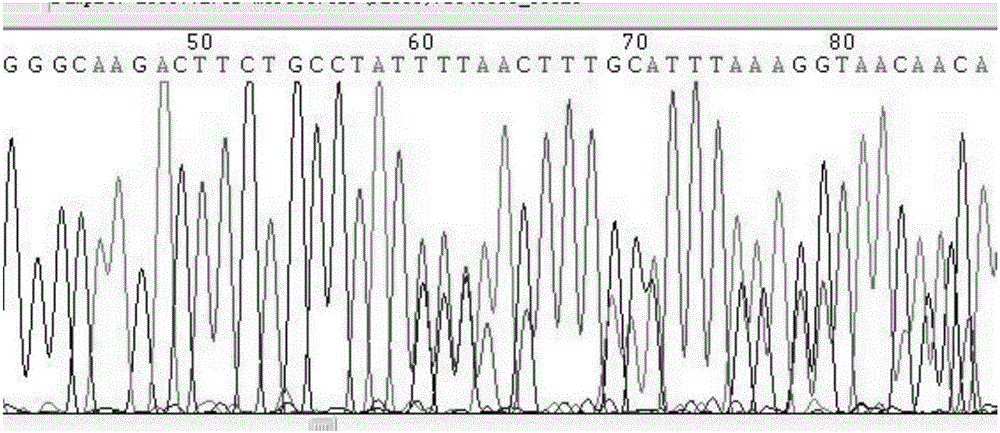
[0082] One case of clinical GIST paraffin tissue samples with known mutation type of exon 9 mutation of c-kit gene (respectively Y503_F504insAH, A501_Y502dup, A502_Y503dup mutation) was selected (for the sequencing results of Y503_F504insAH mutation, see figure 1 ) and a known wild-type clinical GIST paraffin tissue sample to detect the mutation type of human c-kit gene.
[0083] 2. Detection method
[0084] The method of the present invention: adopt the kit of embodiment 1, detect according to the method of embodiment 1.
[0085] Sample DNA extraction method: extract with Qiagen kit:
[0086] 1) Use a scalpel to remove the useless paraffin at the tissue border;
[0087] 2) Cutting the paraffin-embedded tissue into 4 μm thick slices;
[0088] 3) Quickly take 2-6 pieces with sterilized tweezers and put them into DNase / RNase Free ...
experiment example 2
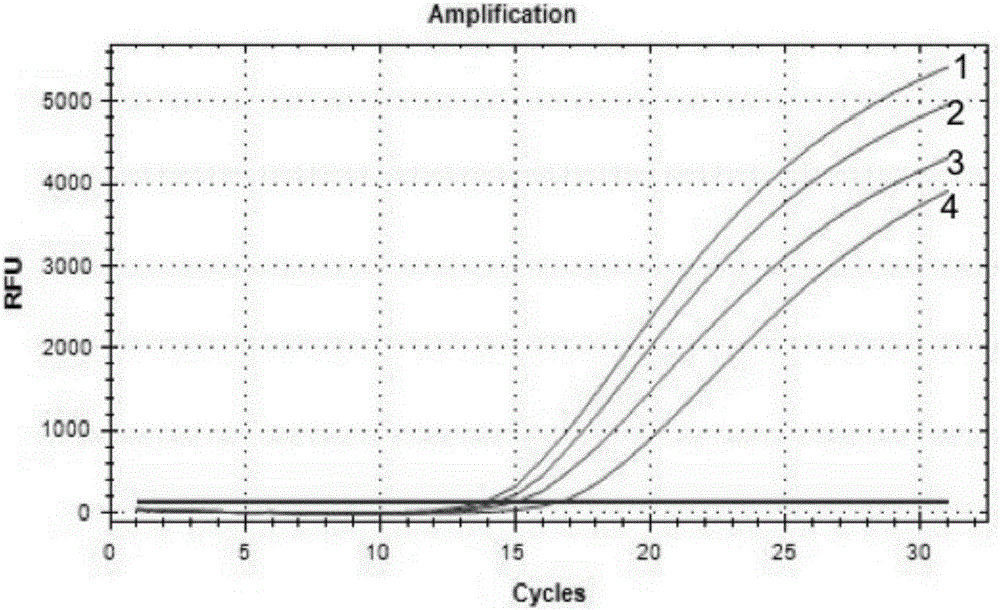
[0107] Experimental example 2 sensitivity analysis of kit of the present invention and method
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com