Stearic acid grafted tetracycline, and preparation and application thereof
A technology of stearic acid and tetracycline, applied in the application field of tetracycline stearic acid graft and its preparation, and bone-targeted lipid nano drug delivery system, which can solve low bioavailability and non-targeted tissue and organ toxic side effects , No bone tissue targeting ability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
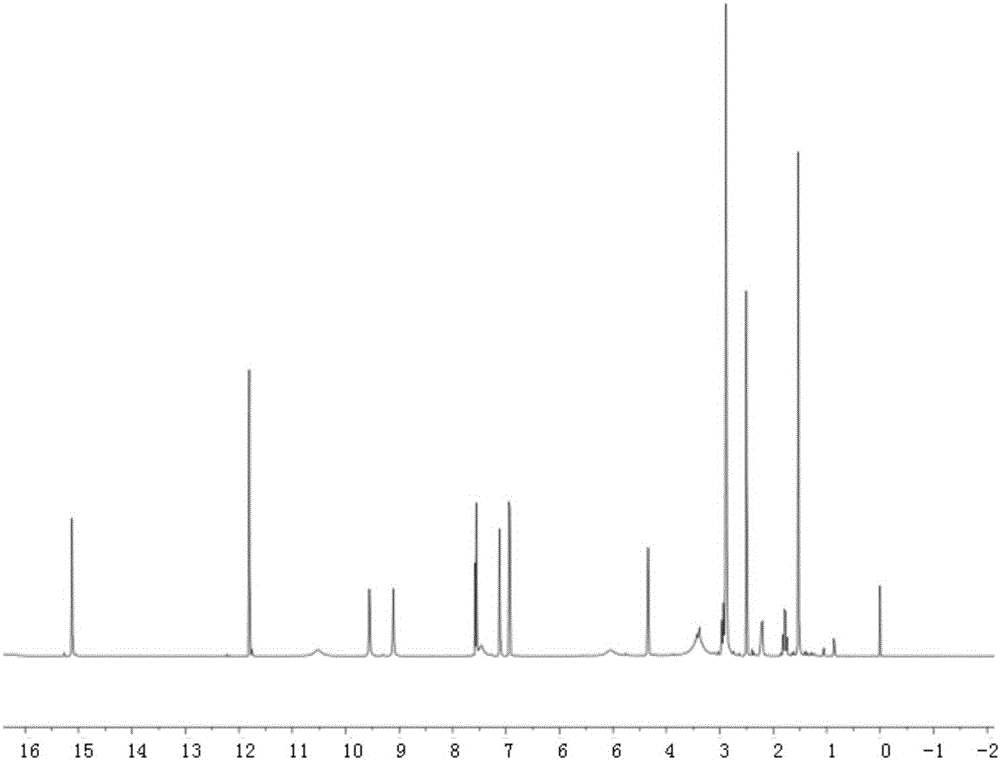
[0020] Embodiment one: the synthesis of tetracycline stearic acid graft
[0021] Tetracycline stearic acid graft is synthesized by chemical reaction between the hydroxyl group of tetracycline and the carboxyl group of stearic acid. Specifically, it is realized through the following steps:
[0022] Accurately weigh 213mg of stearic acid, 216mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide, EDC), 150mg of 1-Hydroxybenzotriazole (Hydroxybenzotriazole, HOBT) was placed in a 100ml dry round bottom flask (the molar ratio of stearic acid, EDC and HOBT was 1:1.5:1.5), and 20ml of anhydrous dimethyl formazan was added Amide, stir at 60°C to dissolve all the reactants, and keep warm for 30min to activate the carboxyl group of stearic acid. Add 469 mg of tetracycline hydrochloride (Tetracycline, TC) (the molar ratio of stearic acid to tetracycline hydrochloride is 1:1.3) in the round bottom flask, and continue to react for 24 hours unde...
Embodiment 2
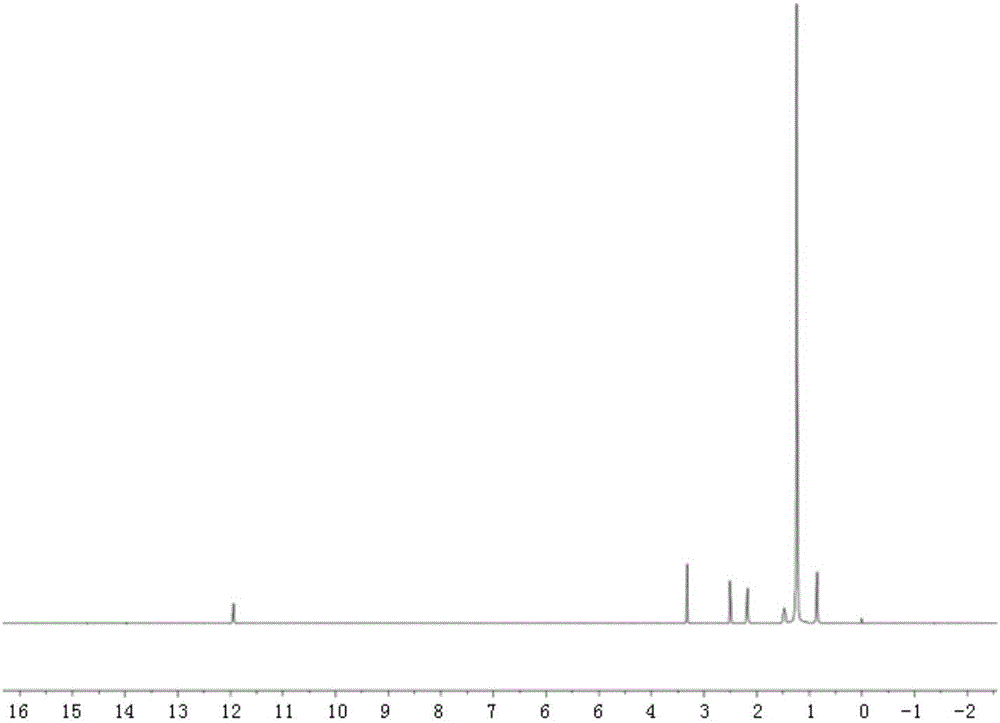
[0025] Example 2: Preparation of bone-targeted lipid nanocarrier containing 30% tetracycline stearic acid graft and its in vitro bone affinity
[0026] 1. Preparation of non-targeting lipid nanocarriers and their in vitro bone affinity.
[0027] Weigh 10 mg of lipid material (one of monoglyceride, stearic acid, glyceryl tristearate, and glyceryl behenate) and dissolve it in 1 ml of fluorescein isothiocyanate stearylamine graft respectively. In water ethanol, heat and dissolve in a water bath at 70°C (glyceryl tristearate and glyceryl behenate are 74°C), and the obtained organic phase is rapidly dispersed at 400rm to the same temperature of 70°C (glyceryl tristearate, glyceryl behenate is 70°C). Glyceryl behenate (74 DEG C) in 10ml poloxamer solution (0.1%, w / v), continued to stir for 5 minutes under water-bath conditions, cooled to room temperature, and obtained non-targeting lipid nanocarrier (being called for short lipid nanocarriers).
[0028] Take 2ml of poloxamer soluti...
Embodiment 3
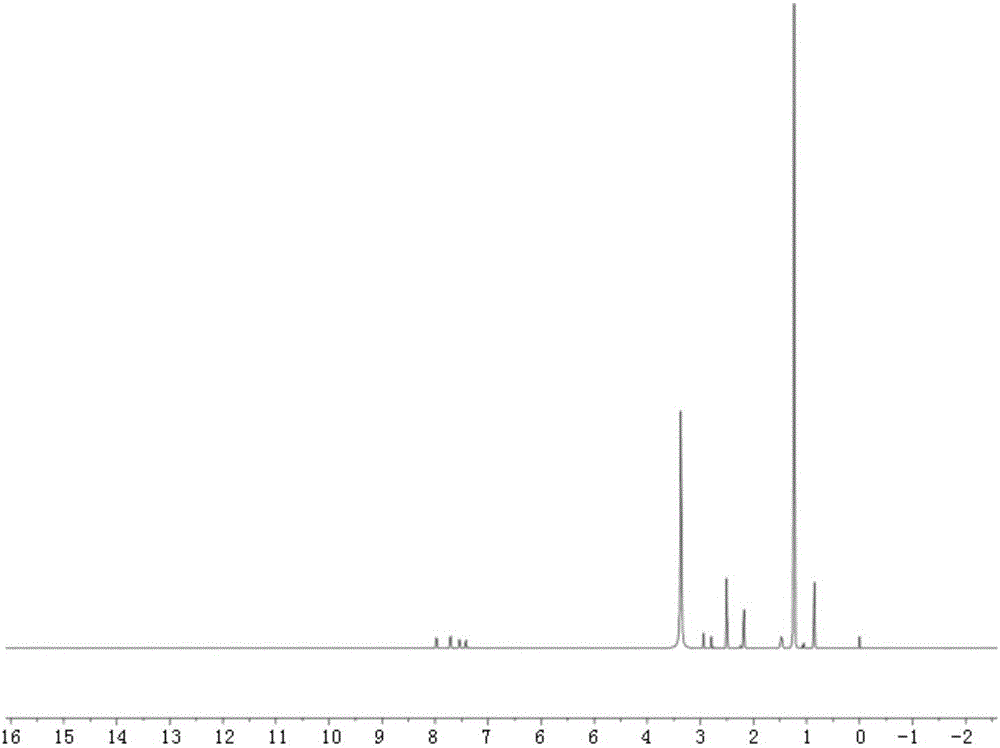
[0035] Example 3. Preparation of bone-targeted lipid nanocarrier containing 50% tetracycline stearic acid graft and its in vitro bone affinity
[0036] Weigh 5 mg of monoglyceride, 1 mg of fluorescein isothiocyanate stearylamine graft and 5 mg of tetracycline stearic acid graft, dissolve in 1 ml of absolute ethanol, heat and dissolve in a water bath at 70 ° C, and obtain the organic phase at 400 rm Rapidly dispersed in 10ml poloxamer solution (0.1%, w / v) at the same temperature of 70°C, continued to stir for 5 minutes under water bath conditions, and cooled to room temperature to obtain the bone-targeting lipid containing tetracycline stearic acid graft nanocarriers.
[0037] Take 2ml of poloxamer solution + 20mg of hydroxyapatite, stir for 1h, centrifuge at 10000r for 5min, take the supernatant + 2ml of bone-targeting lipid nanocarriers and mix evenly to measure the fluorescence value as the control group. Take another 2ml of bone-targeting lipid nanocarrier + 2ml of poloxam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com