Application of tanshinone II A derivative in drugs
A technology of tanshinone and derivatives, which is applied in the field of tanshinone derivatives, and can solve problems such as rarely reported applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
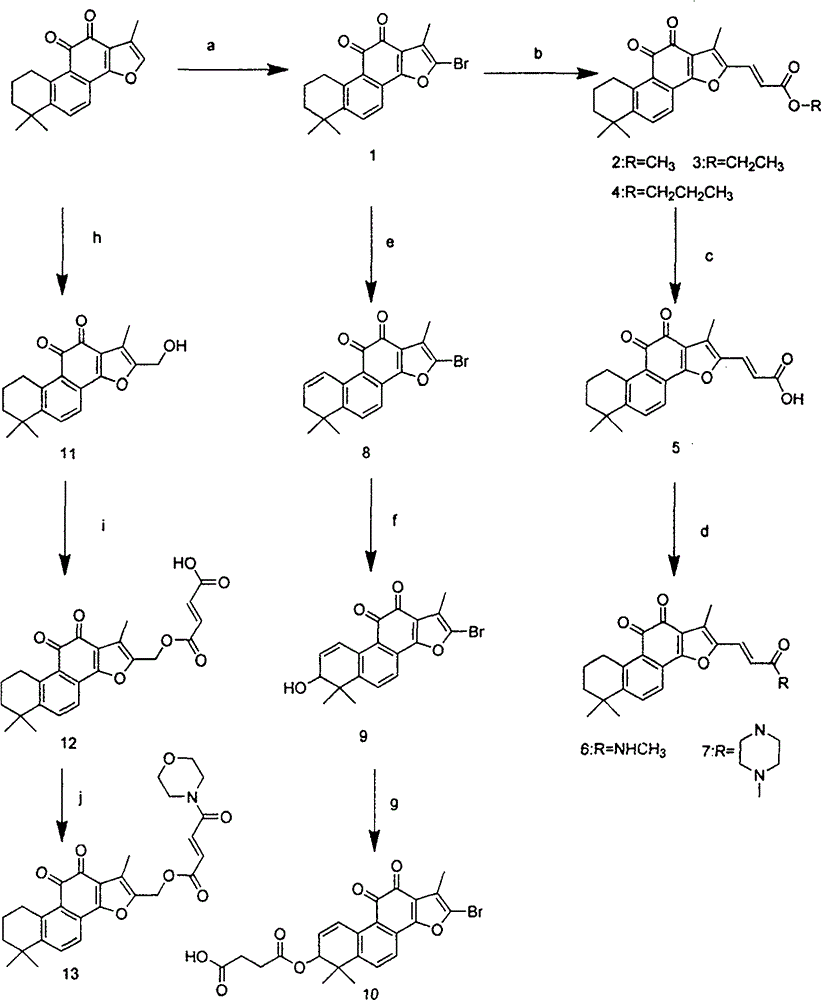
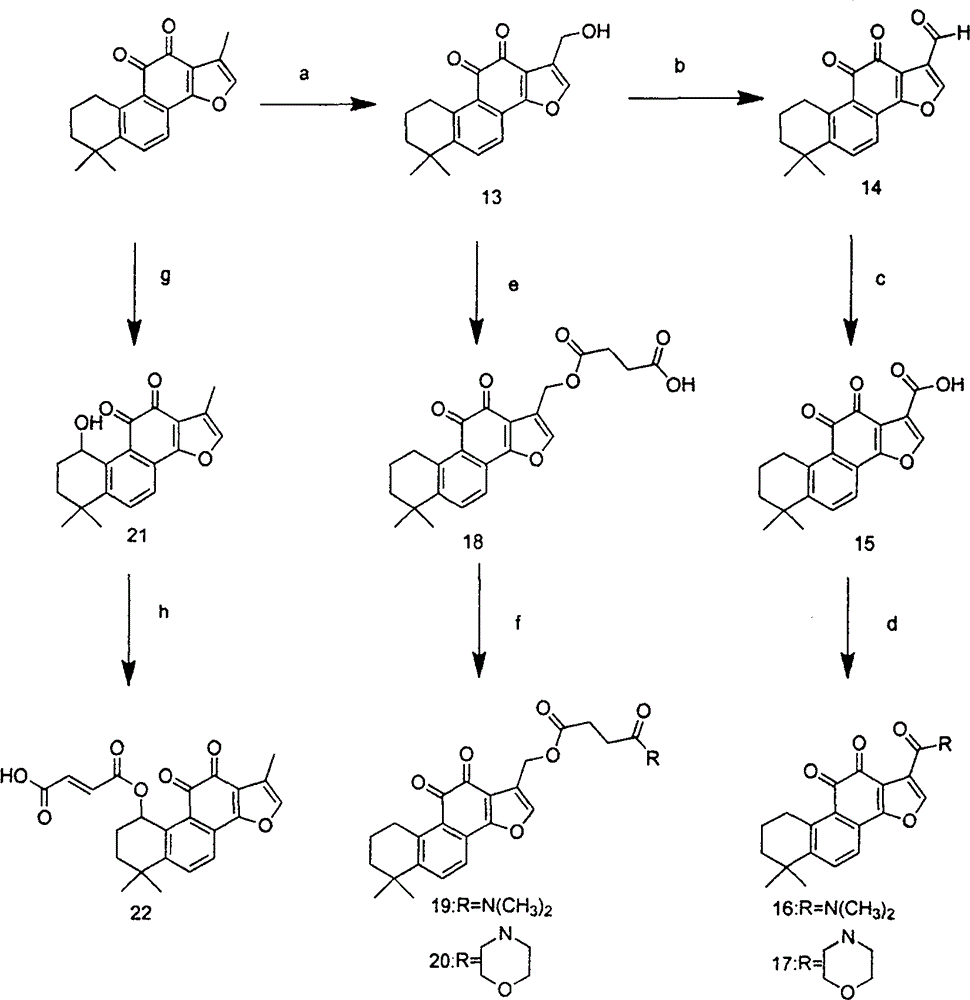
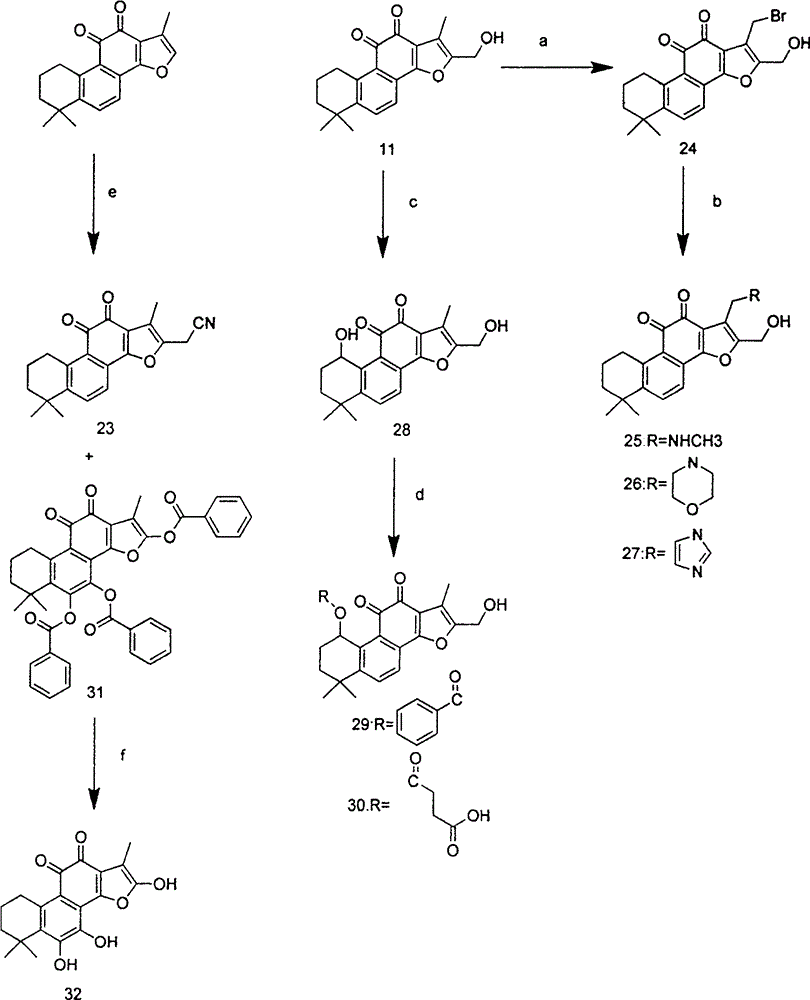
[0052] Preparation of Compounds 3, 5, 7
[0053] Dissolve 580mg (2mmol) of tanshinone IIA in 20mL of dichloromethane, add 0.5mL of hydrobromic acid, add 0.15mL (1.95mmol) of liquid bromine, and react in the dark for 30min. After the reaction stopped, 30 mL of a saturated aqueous solution of sodium bisulfite was added, stirred for 10 min, washed with water, and dried over anhydrous sodium sulfate. Purified by column chromatography (PE:EA=36:1) to obtain compound 1. EI / MS (m / z): 372 (M + ).
[0054] 220mg (0.75mmol) of compound 1 was dissolved in 22mL of DMF, 0.3mL of triethylamine (2.3mmol), 0.16mL (1.5mmol) of ethyl acrylate, 38mg (0.15mol) of triphenylphosphine, 17mg (0.075mmol) of palladium acetate were added , heated at 100°C for 8 hours, after the reaction, filtered with suction to obtain the filtrate, added 20mLEA, washed with water, anhydrous Na 2 SO 4 dry. Purified by column chromatography (PE:EA=24:1) to obtain compound 3. 1 H NMR (300MHz, CDCl 3 ): 1.32 (9H, m...
Embodiment 2
[0058] Preparation of compound 10
[0059] Dissolve 372mg (1mmol) of compound 1 in 20mL of DMF, add 0.3mL of triethylamine (2.3mmol), 380mg (1.5mol) of triphenylphosphine, 170mg (0.75mmol) of palladium acetate, heat at 100°C for 16h and spin dry, add water 30mL, extracted with DCM, washed with water, anhydrous Na 2 SO 4 dry. Purified by column chromatography (PE / EA=36:1) to obtain compound 8.
[0060] Dissolve 140mg (0.38mmol) of compound 4 in 15ml of acetonitrile, heat and stir until dissolved, add selenium dioxide, heat the oil bath to 44°C and heat to reflux for 10h, cool to room temperature, filter with suction to obtain the filtrate, wash with 20ml of 5% NaOH solution 3 times, brine washing, anhydrous Na 2 SO 4 dry. Purified by column chromatography (PE / EA=12:1) to obtain compound 9.
[0061] 31mg (0.1mmol) of compound 5 was dissolved in 4mL of dichloromethane, 15mg (0.15mmol) of maleic anhydride, 0.03mL (0.3mmol) of triethylamine, a catalytic amount of DMAP were a...
Embodiment 3
[0063] Preparation of Compound 12
[0064] 117mg (0.4mmol) of tanshinone IIA was dissolved in 10mL of acetonitrile, 1.2mL (4.4mmol) of formaldehyde was added, 1d of acetic acid was reacted at reflux at 70°C for 30min, and purified by column chromatography (PE:EA=36:1) to obtain compound 11. 1 H NMR (300MHz, CDCl 3 ): 1.32 (6H, s, CH 3 ), 1.65 (2H, m, CH 2 ), 1.77 (2H, m, CH 2 ), 2.31 (3H, s, CH 3 ), 3.19 (2H, t, CH 2 ), 4.65 (2H, s, CH 2 ), 7.63 (2H, dd, Ar).
[0065]65 mg (0.2 mmol) of compound 11 was dissolved in 8 mL of dichloromethane, 60 mg (0.6 mmol) of maleic anhydride and 0.1 mL (0.6 mmol) of triethylamine were added, and stirred at room temperature for 12 h to stop the reaction. Purified by column chromatography (PE:EA=1:2) to obtain compound 12. (KBr)v max (cm -1 ): 2933.53, 2737.67, 2675.96, 2491.11, 1573.67, 1475.38, 1384.59, 1275.40, 1260.65, 1192.28, 875.61, 864.02. 1 H NMR (300MHz, DMSO-D 6 ): 1.32 (6H, s, CH 3 ), 1.65 (2H, m, CH 2 ), 1.77 (2H, m, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com