Tobacco etch virus protease active inclusion body as well as preparation method and application thereof
A technology of tobacco etch virus and protease activity, applied in the biological field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0033] Source of biological material:
[0034] 1. pET-30a vector: imported from Novagen, USA, and preserved in our laboratory.
[0035] 2. Escherichia coli DH5a: imported from BD Biosciences Clontech, USA, and preserved in our laboratory.
[0036] 3. BL21(DE3) Escherichia coli: imported from Novagen, USA, and preserved in our laboratory.
[0037] 4. MDBK cells: imported from ATCC in the United States and preserved in our laboratory.
[0038] 5. Vesicular stomatitis virus (VSV): preserved by our laboratory (document: Xu Yaoxian, Zhou Xiaofeng, Liu Lide. Molecular Virology. Wuhan: Hubei Science and Technology Press, 2000.300-302).
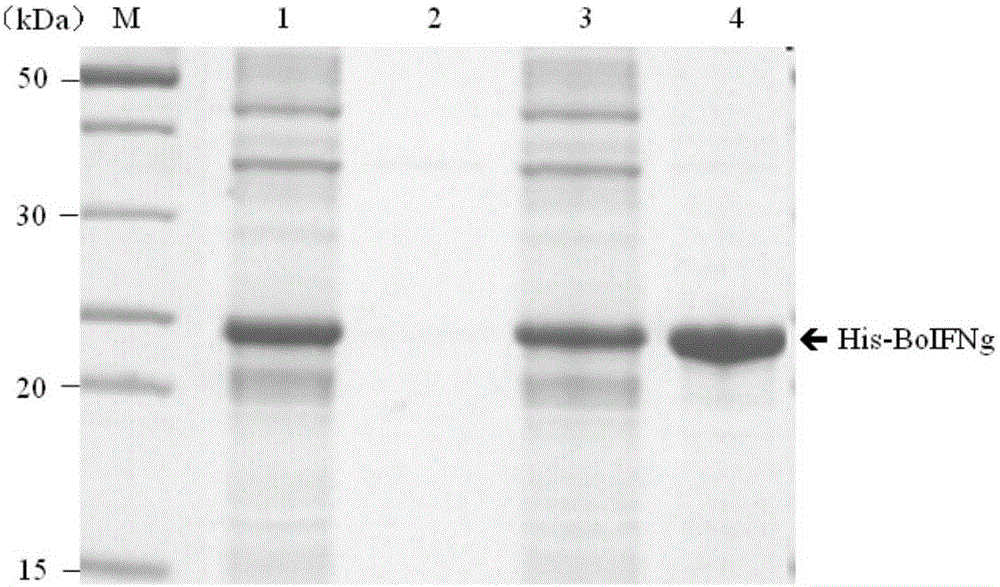
[0039] 6. pGEX-BoIFNg vector: Constructed and preserved by our laboratory (document: Xu Jinjun, Qin Aijian, Jin Wenjie, etc. Cloning of cow gamma interferon gene and its expression in Escherichia coli. Journal of Yangzhou University, 2003, 24(1): 5-10).
[0040] The specific operation steps are as follows:
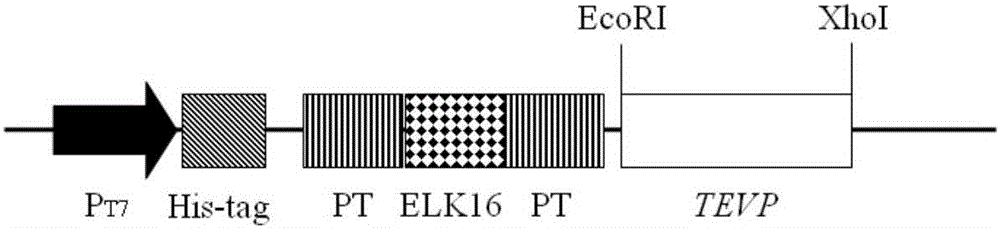
[0041] 1. Construction of pP16P-TEVP ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com