Eph kinase polypeptide inhibitor and application thereof
A technology of kinase inhibition and amino acid, applied in the biological field, to achieve the effect of inhibiting tumor angiogenesis, high stability, and efficient inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1 Polypeptide compound of the present invention and its synthesis
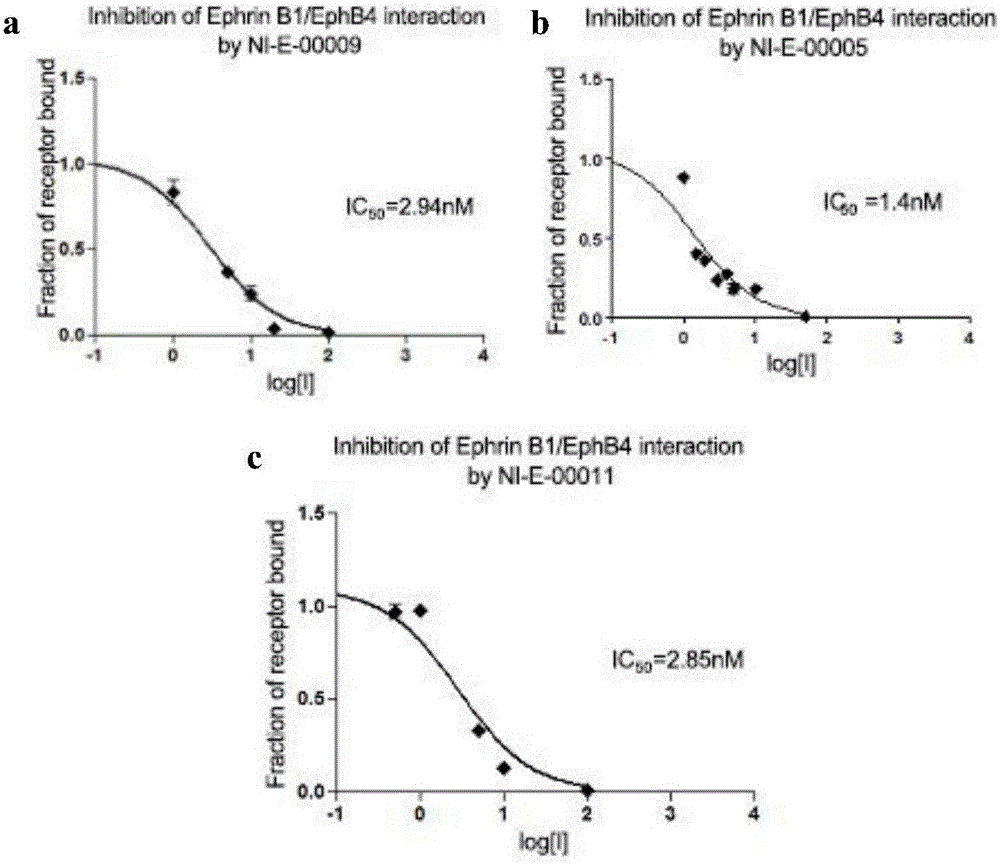
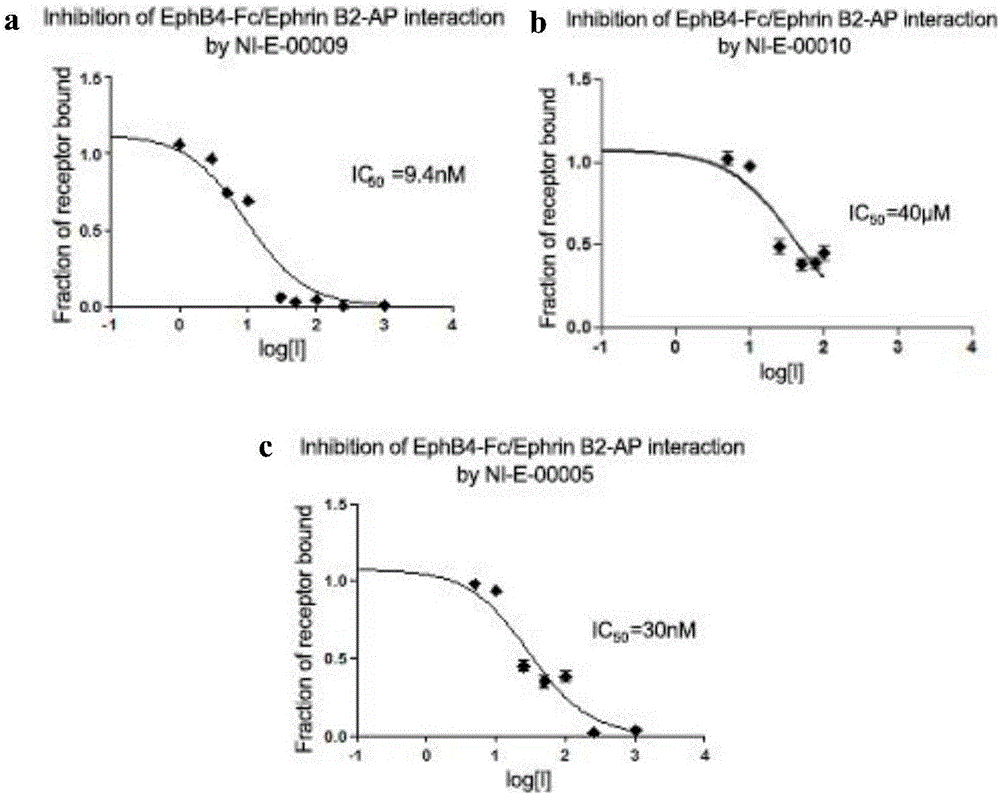
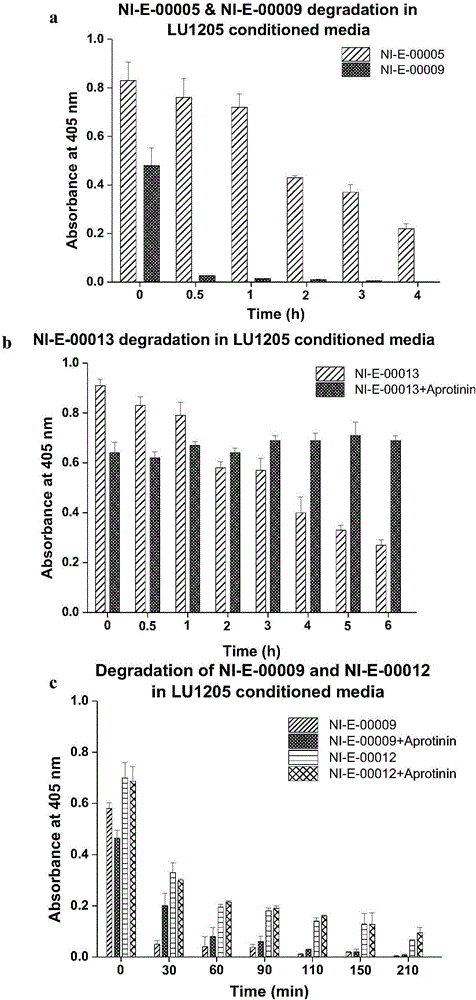
[0045] The amino acid sequence of Eph kinase polypeptide inhibitor NI-E-00005 is: Tyr-Asn-Tyr-Leu-Phe-Ser-Pro-Asn-Gly-Pro-Ile-Ala-His-Ala-Trp-NH 2 (SEQ ID NO:5), synthesized by the following route.
[0046]
[0047] 1. It is synthesized by Fmoc solid-phase synthesis method.
[0048] Weigh 714mg of Rink Amide-MBHA resin with a substitution degree of 0.28mmol / g in a solid phase reactor, add DCM to swell for 30min, then add 20% PIP / DMF solution for deprotection twice, the first time is 5min, and the second time is 20min . Wash several times with DCM and DMF respectively, and drain. Add Fmoc-Trp(Boc)-OH (426mg, 1mmol), HOBt (135mg, 1mmol), DIC (126mg, 1mmol), DMF (6mL) into the solid phase reactor, and react at room temperature for 2h. Wash and dry the resin to obtain Fmoc-Trp(Boc)-Rink Amide-MBHA resin. Add 20% PIP / DMF solution for deprotection twice, the first time is 5 minutes, the sec...
Embodiment 2
[0051] Embodiment 2 Polypeptide compound of the present invention and synthesis
[0052] Amino acid sequence of Eph kinase polypeptide inhibitor NI-E-00013 Tyr-Asn-Tyr-Leu-Phe-Ser-Pro-Asn-Gly-Pro-Ile-Ala-His-Ala-Trp-Ahx-Lys(Biotin)- NH 2 (SEQ ID NO:6), synthesized by the following route.
[0053]
[0054] 1. It is synthesized by Fmoc solid-phase synthesis method.
[0055] Weigh 357 mg of Rink Amide-MBHA resin with a substitution degree of 0.28 mmol / g in a solid phase reactor, add DCM to swell for 30 min, then add 20% PIP / DMF solution for deprotection twice, and react for 5 min and 20 min respectively. Wash several times with DCM and DMF respectively, and drain. Add Fmoc-Lys(Biotin)-OH (297.4mg, 0.5mmol), HOBt (67.5mg, 0.5mmol), DIC (63mg, 0.5mmol), DMF (4mL) to the solid phase reactor, and react in the dark for 2h at room temperature . Wash and dry the resin to obtain Fmoc-Lys(Biotin)-Rink Amide-MBHA resin. Then add 20% PIP / DMF solution twice for deprotection, and rea...
Embodiment 3
[0058] Embodiment 3 The polypeptide compound of the present invention
[0059] The amino acid sequence of the Eph kinase polypeptide inhibitor is shown in Table 1, and was synthesized according to the method described in Example 1 or Example 2.
[0060] Table 1 Eph Kinase Polypeptide Inhibitors
[0061]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com