Method used for synthesizing heterocycle substituted multi-fluoro arene through defluorination and hydrogenation and controlled by phosphine ligand
A phosphine ligand and hydrogenation technology, which is applied in the chemical industry and can solve problems such as unstable hydrogenation of intermediates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
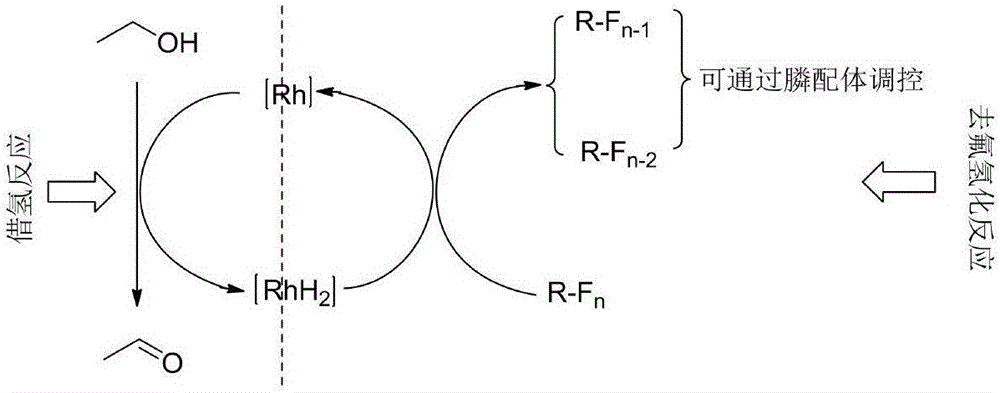
[0034] For rhodium catalyzed dehydrofluorination, the inventor proposes conjecture ( figure 1 ): ethanol is used as a hydrogen source, and the hydrogen on the ethanol is transferred to metal rhodium through a hydrogenation reaction to form a metal rhodium hydride. The rhodium hydride is then used as a catalytic intermediate for dehydrofluorination to complete the dehydrofluorination reaction. During this period, through the control of ligands, monofluorodehydrofluorination products or bisdehydrofluorination products are selectively obtained.
[0035] Therefore, the rhodium complex ([Cp*RhCl 2 ] 2 , [(COD)RhCl] 2 ) as a catalyst to explore the optimal reaction conditions for dehydrofluorination. 2-Perfluorophenylpyridine as a model substrate for the reaction. Pinacol diboronic acid ester was added to the reaction system as a fluorine atom capture agent. Ethanol was used as the reaction solution, the reaction temperature was set at 90° C., and the reaction time was 12 h. ...
Embodiment 2
[0041] Example 2: Mono dehydrofluorination
[0042] After exploring the reaction conditions, the inventors investigated the applicability of the reaction using the conditions.
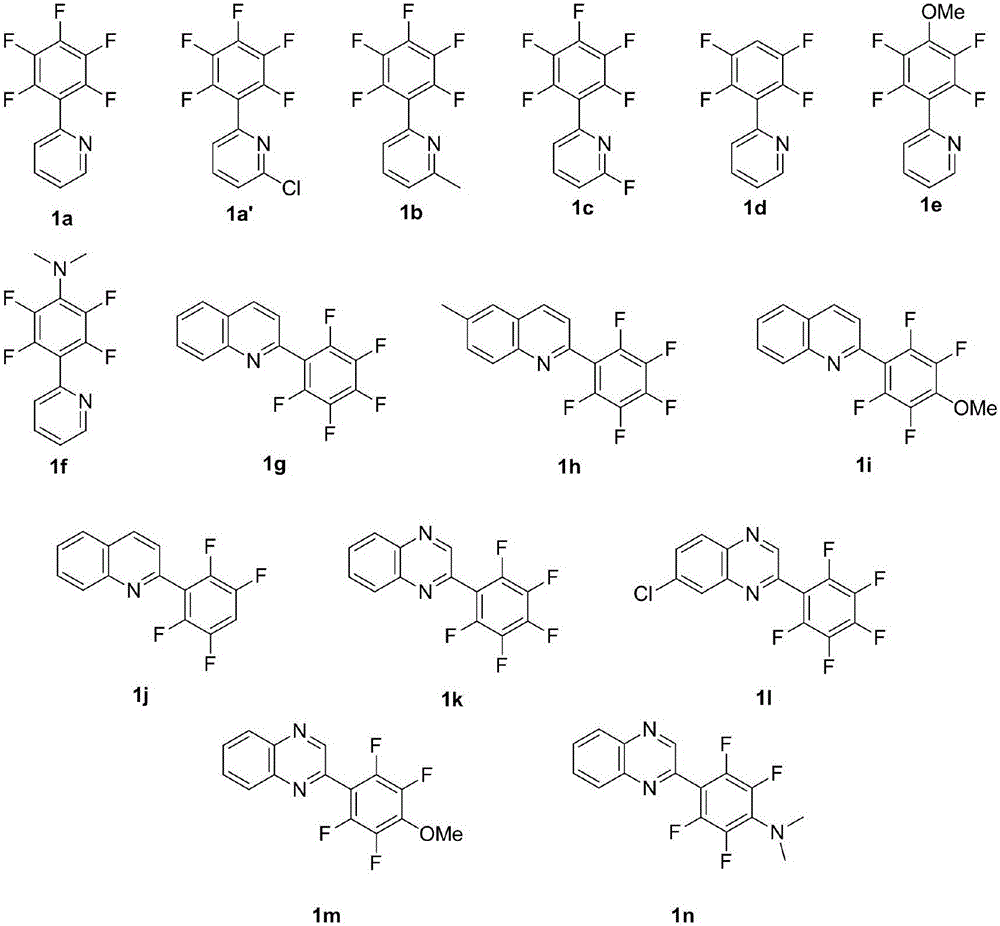
[0043] Firstly, a series of nitrogen-containing heterocyclic substituted polyfluorinated aromatic hydrocarbons were synthesized, such as figure 2 shown.
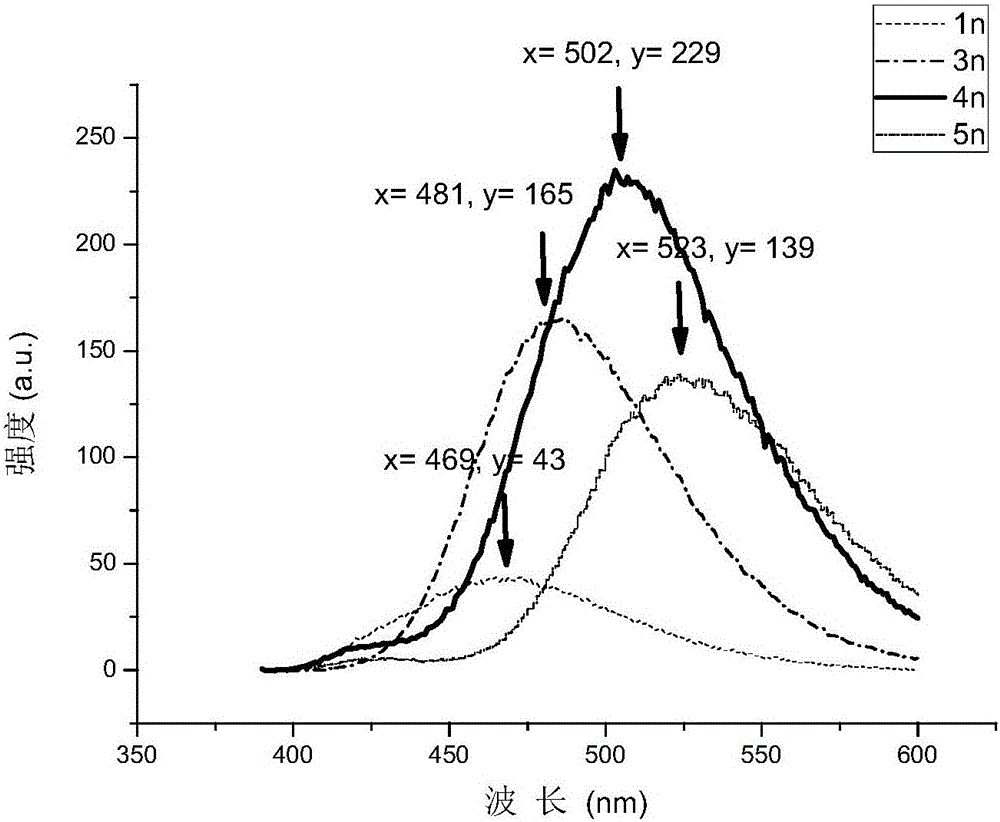
[0044] Using DPPE as a ligand, the monodehydrofluorination reaction was studied ( image 3 ). Wherein the reaction conditions are as follows: Compound 1 (0.25mmol), [(COD)RhCl] 2 (0.0125mmol), DPPE (0.025mmol), AgBF 4 (0.025mmol), B 2 pin 2 (0.5mmol), KOAc (0.5mmol), solvent (toluene:ethanol=4:1) 2.0mL.
[0045] It was found that the substrates substituted with perfluorobenzene could be separated to obtain the target product in higher yield ( Figure 4 3a, 3a', 3b, 3c, 3g, 3h, 3k, 3i). Among them, 3b and 3c, in the presence of C-X (C, F) at the ortho position of the pyridine ring, the reaction still maintains a good yield. When the ortho p...
Embodiment 3
[0046] Example 3: Double dehydrofluorination
[0047] Using Xant-phos as the phosphine ligand, the applicability of this method to double defluorination reactions was investigated ( Figure 5 ). Wherein the reaction conditions are as follows: Compound 1 (0.25mmol), [(COD)RhCl] 2 (0.0125mmol), Xant-phos(0.025mmol), AgBF 4 (0.025mmol), B 2 pin 2 (0.5mmol), KOAc (0.5mmol), solvent (toluene:ethanol=4:1) 2.0mL.
[0048] The reaction is similar to that of monodehydrofluorination. It is worth mentioning that when using image 3 When the monofluorinated defluorinated product (compound 3) in is used as a raw material to obtain double defluorinated product, the yield ratio Figure 6 The listed yields are slightly higher.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com