A kind of preparation method of amino protecting group
A technology of amino protecting group and chloromethyl group, which is applied in the field of preparation of amino protecting group, can solve the problems of acid sensitivity, alkali instability, and many reaction by-products, and achieve high yield, large application value, and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
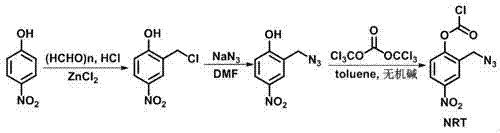
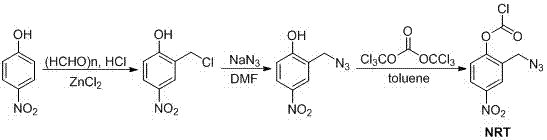
[0029] Preparation of 2-chloromethyl-4-nitrophenol: 4-nitrophenol (13.9 g, 0.1 mol), paraformaldehyde (6.0 g, 0.2 mol), anhydrous zinc chloride (0.1 g), 100 Add mL of concentrated hydrochloric acid into a 250 mL three-neck flask, raise the temperature to 70°C and start to pass hydrogen chloride gas, and keep it warm for 6 h, filter, and recrystallize with chloroform to obtain 14.0 g of white solid with a yield of 75%.
[0030] 2-Azidomethyl-4-nitrophenol: Add 2-chloromethyl-4-nitrophenol (5.6 g, 0.03 mol) with sodium azide (3.9 g, 0.06 mol) to 30 mL of DMF , reacted at 40°C for 12 h, cooled to room temperature, added 80 mL of water, extracted with dichloromethane, dried, and concentrated in vacuo to obtain 5.7 g of reddish-brown liquid with a yield of 98%.
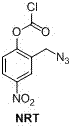
[0031] Amino-protecting group (NRT) preparation: 2-azidomethyl-4-nitrophenol (2.91 g, 0.015 mol) was mixed with K 2 CO 3 (6.2 g, 0.045 mol) was added to toluene, reacted for 20 min, triphosgene (1.48 g, 0.005 mol) was ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com