Hydrochlorides of dipeptide compounds and preparation method thereof
A compound and hydrochloride technology, applied in the field of chemical synthesis, can solve the problems of easy moisture absorption, not particularly good properties, low product purity, etc., and achieve the effect that it is not easy to absorb moisture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
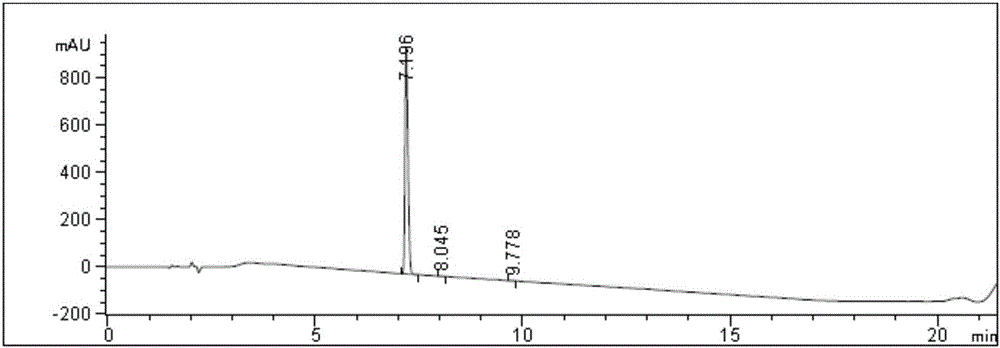
Embodiment 1
[0061] a. Synthesis of crude solution of compound 1
[0062] Add 42.0 g (0.1 mol) of the compound shown in Formula 2 and 168.0 g (2.0 mol) of dichloromethane into a 2000 mL reaction flask, and stir to dissolve at room temperature. Mole / liter of hydrogen chloride dioxane solution 150.0ml (0.6mol), control the temperature at 5-10°C, stir for 3.0 hours, HPLC monitors the remaining 0.07% of the raw material, the reaction is qualified, add 840.0 methyl tert-butyl ether to the reaction solution g (9.5mol), stirred for 10 minutes, poured off the supernatant, added 504.0g (5.7mol) of dioxane at 0-5°C, stirred for 10 minutes, then added 504.0g of methyl tert-butyl ether ( 5.7 mol), stirred at 0-5°C for 30 minutes, and suction filtered to obtain 34.1 g of a crude product with a purity of 98.8% and a yield of 95.7%. b, the purification of formula 1 finished product
[0063] Add 504.0 g (7.0 mol) of tetrahydrofuran to the crude product obtained above, stir at room temperature to dissolv...
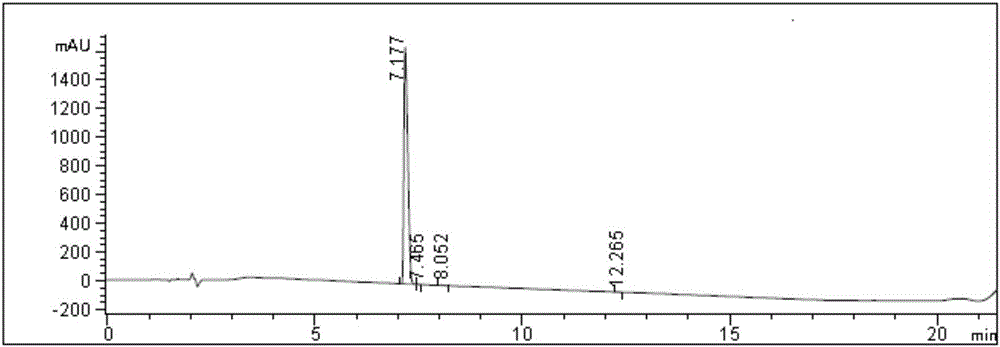
Embodiment 2
[0065] a, the synthesis of compound crude product solution shown in formula 1
[0066] Add 42.0g (0.1mol) of the compound shown in formula 2 and 420.0g (4.9mol) of dichloromethane into a 2000mL reaction bottle, stir and dissolve at room temperature, after the reaction solution is dissolved and clarified, cool down to -2~5°C, and start adding dropwise 125.0ml (0.5mol) of 4 mol / liter hydrogen chloride dioxane solution, control the temperature at 5-10°C, stir for 3.0 hours, HPLC monitors the remaining 1.8% of raw materials, and the reaction is qualified, and methyl tert-butyl ether is added to the reaction solution 840.0g (9.5mol), stirred for 10 minutes, poured off the supernatant, added 504.0g (5.7mol) of dioxane at 0-5°C, stirred for 10 minutes, then added 504.0g of methyl tert-butyl ether (5.7mol), stirred at 0-5°C for 30 minutes, and filtered with suction to obtain 30.1 g of crude product with a purity of 96.8% and a yield of 84.2%.
[0067] b. Purification of the finished ...
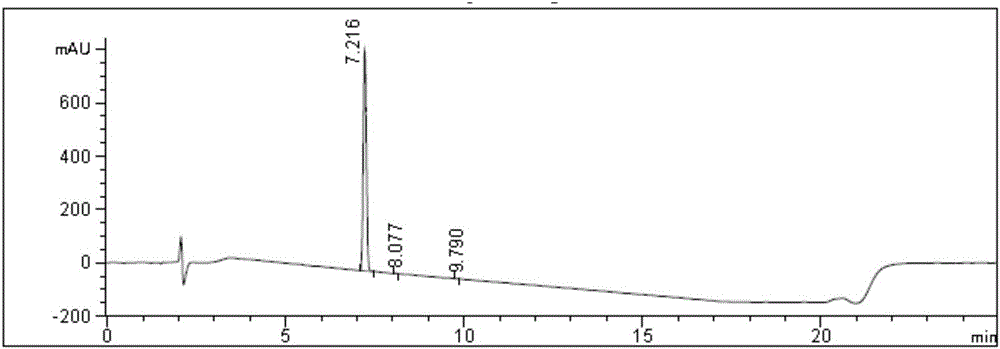
Embodiment 3
[0070] a, the synthesis of compound crude product solution shown in formula 1
[0071] Add 42.0g (0.1mol) of the compound shown in Formula 2 and 168.0g (2.0mol) of dichloromethane into a 2000mL reaction flask, stir and dissolve at room temperature, after the reaction solution is dissolved and clarified, cool down to 5-10°C, and start to add 2 Mole / liter hydrogen chloride dioxane solution 300.0ml (0.6mol), control the temperature at 5-10°C, stir for 3.0 hours, HPLC monitors the remaining 0.3% of raw materials, the reaction is qualified, add 840.0g of methyl tert-butyl ether to the reaction solution (9.5mol), stirred for 10 minutes, poured off the supernatant, added 504.0g (5.7mol) of dioxane at 5-10°C, stirred for 10 minutes, then added 504.0g (5.7mol) of methyl tert-butyl ether mol), stirred at 5-10° C. for 30 minutes, and suction filtered to obtain 32.1 g of a crude product with a purity of 98.0% and a yield of 90.1%.
[0072] b. Purification of the finished product of Compo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com