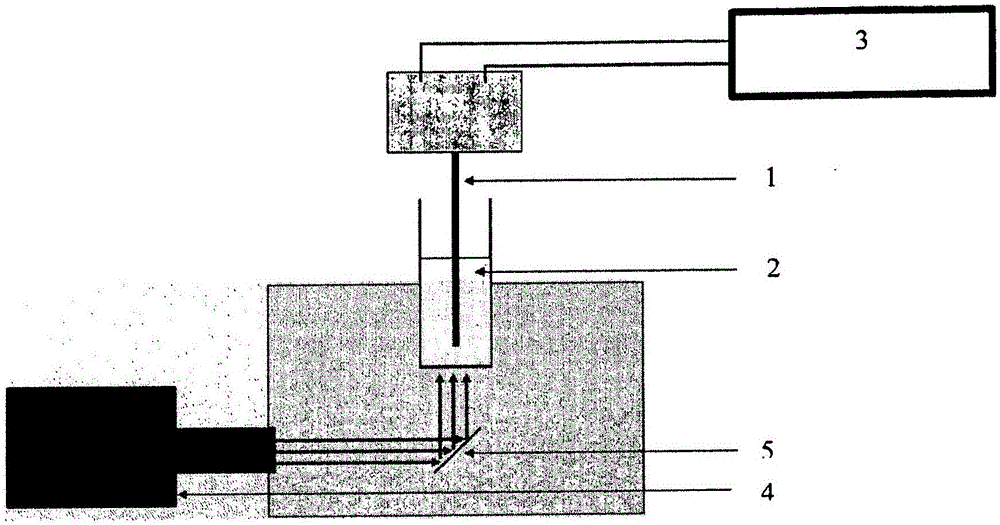
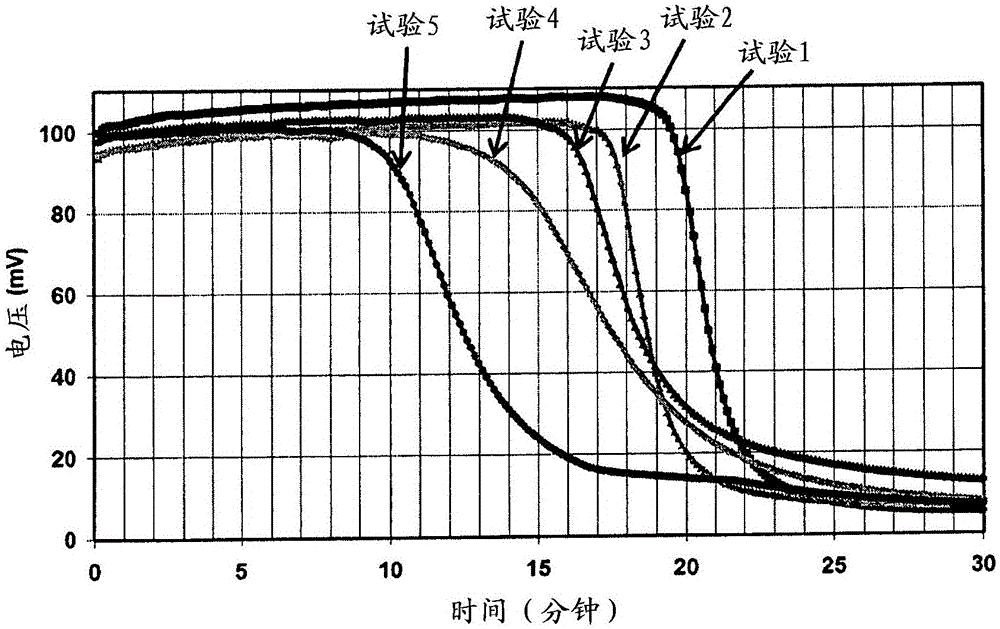
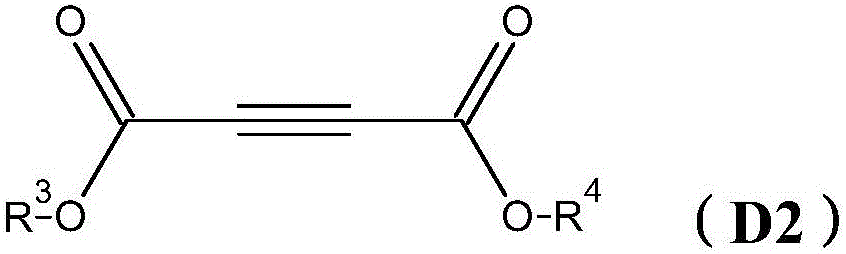
Novel photoactivatable system for inhibiting hydrosilylation
A technology of inhibitor and polysiloxane, which is applied in the direction of coating, etc., can solve the problems of slow curing rate, reduced coating rate, strong inhibition of hydrosilylation catalyst, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0103] Preferably, the polyorganosiloxanes A according to the invention carry at least two alkenyl groups bonded to silicon atoms. According to a preferred embodiment, this polyorganosiloxane A comprises:
[0104] (i) at least two units of formula (A1)
[0105] Y a Z b SiO (4-(a+b) / 2 (A1)
[0106] in:
[0107] -Y represents a monovalent group comprising 2 to 12 carbon atoms having at least one olefinic function and optionally at least one heteroatom,
[0108] -Z represents a monovalent group containing 1-20 carbon atoms without an alkene or alkyne functional group;
[0109] - a and b represent integers, a is 1, 2 or 3, b is 0, 1 or 2 and (a+b) is 1, 2 or 3;
[0110] (ii) and optionally other units of formula (A2):
[0111] Z c SiO (4-c) / 2 (A2)
[0112] in:
[0113] -Z has the same meaning as above, and
[0114] -c represents an integer of 0-3.
[0115] It is to be understood that in formula (A1) above as well as formula (A2), if there are multiple Y and Z group...
Embodiment
[0197] Reagents used:
[0198] POS Vi A: Chain-end vinylated polydimethylsiloxane oil having the average formula M vi D. 75 m vi and dynamic viscosity at 25°C = 100mPa.s, where M Vi =(CH 3 ) 2 (Vinyl)SiO 1 / 2 and D=(CH 3 ) 2 SiO 2 / 2 .
[0199] POS H B: polymethylhydrogensiloxane oil with trimethylsilyl terminations, having the average formula M 2 D' 45 And the dynamic viscosity at 25°C is 20mPa.s, where M=(CH 3 ) 3 SiO 1 / 2 and D'=H(CH 3 ) 1 SiO 2 / 2 .
[0200] Catalyst C: solution of platinum 0 with divinyltetramethyldisiloxane ligands diluted in silicone oil, in which the content of the element Pt is 2800 ppm by mass.
[0201] Inhibitor D: 1-ethynyl-1-cyclohexanol (ECH), which is a true (vrai) α-acetylenic alcohol.
[0202] Photoinitiator E:
[0203] - Omnirad 102, marketed by the company IGM Resins: 2-Hydroxy-2-methyl-1-(4-tert-butyl)phenylacetone (type I photoinitiator);
[0204] - TZT, marketed by the company Lamberti: a mixture of 4-methylbenzophe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com