Method for producing alicyclic polycarboxylic acid
A polycarboxylic acid, alicyclic technology, applied in the direction of ozone oxidation to prepare carboxylic acid, organic chemistry, etc., can solve the problems of precipitation, adhesion, heat transfer efficiency reduction, etc., and achieve the effect of simple temperature control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070]
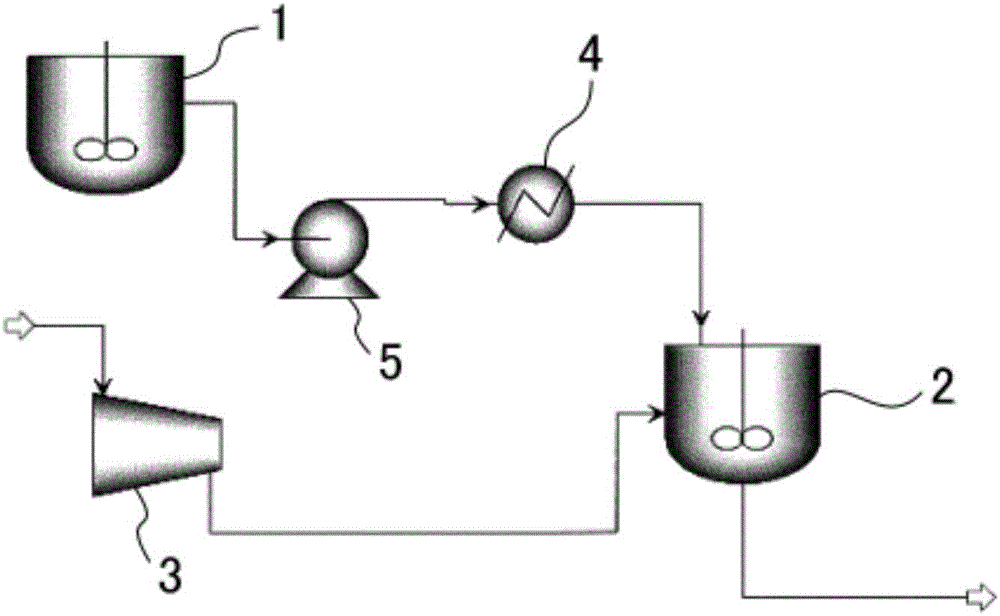
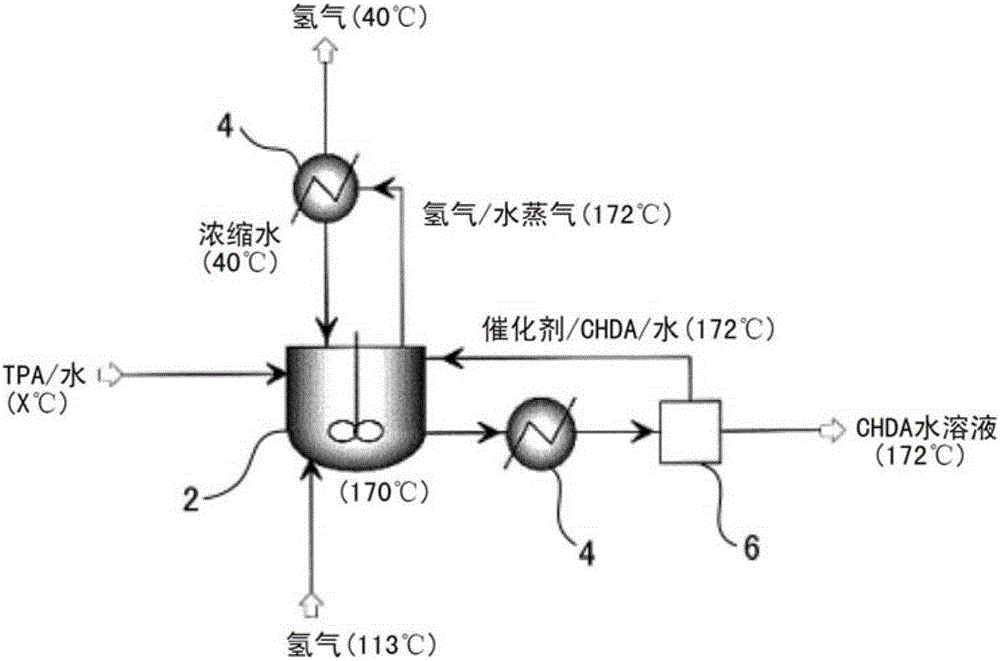
[0071] use as figure 1 In the reaction device with the shown configuration, terephthalic acid (TPA) is hydrogenated to prepare 1,4-cyclohexanedicarboxylic acid (CHDA).
[0072] First, in a raw material preparation tank at normal temperature, 1599 kg / h of TPA at 20°C was mixed and dispersed in 6397 kg / h of water at 20°C to prepare a slurry with a TPA concentration of 20% by weight (TPA / water (weight ratio)=20 / 80).
[0073] The pressure was raised to 6.1 MPa G (gauge pressure, the same below), and the above-mentioned slurry was supplied to a reactor with a stirrer at a supply rate of 7996 kg / h. A heat exchanger regulates the temperature to 91°C and maintains it at 170°C.
[0074] In the reactor, the catalyzer (Pd / C catalyzer) of supporting palladium on the activated carbon of 2% by weight is flowing, while maintaining 6.1MPa G in the stirring tank, with 2664Nm 3 / h Hydrogen gas (6.1 MPa·G) at 114°C was supplied to the reactor.
[0075] While filtering the Pd / C ca...
Embodiment 2
[0106] It was carried out in the same manner as in Example 1 except that the following points were changed.
[0107] That is, in a raw material preparation tank at room temperature, 800 kg / h of TPA at about 20°C was mixed and dispersed in 7196 kg / h of water at 20°C to prepare a slurry with a TPA concentration of 10% by weight (TPA / water (weight ratio) = 10 / 90). The pressure is increased to 6.1MPa·G (gauge pressure, the same below) by hydrogen gas, and the slurry is supplied to the reactor with a stirrer at a supply rate of 7996kg / h. The heat exchanger regulates the temperature to 113°C and maintains it at 170°C.
[0108] In the reactor, flow the catalyzer (Pd / C catalyst) of palladium on the active carbon of 2% by weight, keep 6.1MPa G in the stirred tank simultaneously, with 1342Nm 3 / h Hydrogen gas (6.1 MPa·G) at 74°C was supplied to the reactor. While filtering the Pd / C catalyst, 160,789kg / h of reaction product liquid and 951Nm of hydrogen containing saturated water vapor...
Embodiment 3
[0125] It was carried out in the same manner as in Example 1 except that the following points were changed.
[0126] That is, in a raw material preparation tank at room temperature, 2399 kg / h of TPA at about 20° C. was mixed and dispersed in 5,597 kg / h of water at 20° C. to prepare a slurry with a TPA concentration of 30% by weight (TPA / water (weight ratio) = 30 / 70). The pressure is increased to 6.1MPa·G (gauge pressure, the same below) by hydrogen gas, and the slurry is supplied to the reactor with a stirrer at a supply rate of 7996kg / h. The heat exchanger regulates the temperature to 58°C and maintains it at 170°C.
[0127] In the reactor, the catalyzer (Pd / C catalyst) of supporting palladium on the activated carbon of 2% by weight is flowing, while maintaining 6.1MPa G in the stirring tank, with 3990Nm 3 / h Hydrogen gas (6.1 MPa·G) at 128°C is supplied to the reactor. While filtering the Pd / C catalyst, the reaction product liquid 161924kg / h and the hydrogen gas 2391Nm co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com