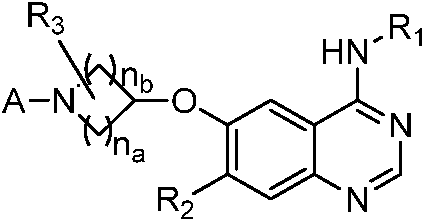
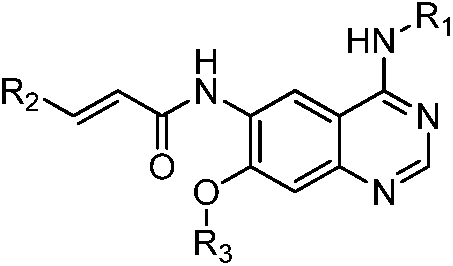
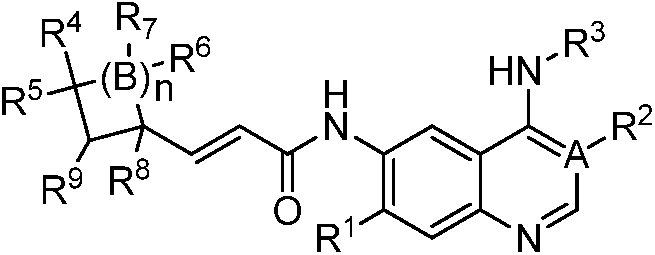
Quinazoline derivatives and their preparation methods and applications in medicine
A pharmacy and drug technology, applied in the field of quinazoline derivatives and their preparation, to achieve the effect of excellent pharmacodynamic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0148] Example 1: 1-[4-[[4-(3-chloro-2,4-difluoro-anilino)-7-methoxy-quinazolin-6-yl]oxymethyl]-1 -piperidinyl]propyl-2-en-1-one (compound 1)
[0149] 1-[4-[[4-(3-chloro-2,4-difluoro-anilino)-7-methoxy-quinazolin-6-yl]oxymethyl]-1-piperidyl]prop-2-en-1-one
[0150]
[0151] The first step: tert-butyl 4-[(4-chloro-7-methoxy-quinazolin-6-yl)oxymethyl]piperidine-1-carboxylate (1B)
[0152] tert-butyl4-[(4-chloro-7-methoxy-quinazolin-6-yl)oxymethyl]piperidine-1-carboxylate
[0153] 1d (3.0g, 14.2mmol), triphenylphosphine (5.58g, 21.3mmol) and dichloromethane (50mL) were added to the reaction flask, protected by nitrogen, cooled to 0°C in an ice bath, and diazobiscarboxylic acid was added dropwise A solution of isopropyl ester (4.30 g, 21.3 mmol) in dichloromethane (10 mL) was added dropwise and allowed to react at room temperature for 30 minutes. 4-Hydroxymethylpiperidine-1-carboxylic acid tert-butyl ester (4.58 g, 21.3 mmol) was slowly added at 0° C., and the mixture was ra...
Embodiment 2
[0167] Example 2: 1-[4-[4-(3-chloro-2,4-difluoro-anilino)-7-methoxy-quinazolin-6-yl]oxy-3-fluoro-1 -piperidinyl]propyl-2-en-1-one (compound 2, compound 2 is 1-[(3R,4R)-4-[4-(3-chloro-2,4-difluoro-aniline) -7-methoxy-quinazolin-6-yl]oxy-3-fluoro-1-piperidinyl]propyl-2-en-1-one and 1-[(3S,4S)-4- [4-(3-Chloro-2,4-difluoro-aniline)-7-methoxy-quinazolin-6-yl]oxy-3-fluoro-1-piperidinyl]propyl-2- mixture of en-1-ones)
[0168] 1-[4-[4-(3-chloro-2,4-difluoro-anilino)-7-methoxy-quinazolin-6-yl]oxy-3-fluoro-1-piperidyl]prop-2-en-1- one
[0169]
[0170]
[0171] The first step: 4-[4-(3-chloro-2,4-difluoro-anilino)-7-methoxy-quinazolin-6-yl]oxy-3-fluoro-piperidine-1 - tert-butyl formate (2B, 2B is (3R,4R)-4-[4-(3-chloro-2,4-difluoro-aniline)-7-methoxy-quinazolin-6-yl] Oxy-3-fluoro-piperidine-1-carboxylic acid tert-butyl ester and (3S,4S)-4-[4-(3-chloro-2,4-difluoro-aniline)-7-methoxy-quinone Azolin-6-yl]oxy-3-fluoro-piperidine-1-carboxylic acid tert-butyl ester mixture)
[0...
Embodiment 3
[0187]Example 3: 1-[4-[4-(3-chloro-2,4-difluoro-aniline)-7-methoxy-quinazolin-6-yl]oxy-3-fluoro-1- Piperidinyl] propyl-2-en-1-one (compound 3, compound 3 is 1-[(3R, 4S)-4-[4-(3-chloro-2,4-difluoro-aniline)- 7-methoxy-quinazolin-6-yl]oxy-3-fluoro-1-piperidinyl]propyl-2-en-1-one and 1-[(3S,4R)-4-[ 4-(3-Chloro-2,4-difluoro-aniline)-7-methoxy-quinazolin-6-yl]oxy-3-fluoro-1-piperidinyl]propyl-2-ene -1-ketone mixture)
[0188] 1-[4-[4-(3-chloro-2,4-difluoro-anilino)-7-methoxy-quinazolin-6-yl]oxy-3-fluoro-1-piperidyl]prop-2-en-1- one
[0189]
[0190] The first step: 4-[4-(3-chloro-2,4-difluoro-aniline)-7-methoxy-quinazolin-6-yl]oxy-3-fluoro-piperidine-1- tert-butyl formate (3B, 3B is (3R, 4S)-4-[4-(3-chloro-2,4-difluoro-aniline)-7-methoxy-quinazolin-6-yl]oxy tert-butyl-3-fluoro-piperidine-1-carboxylate and tert-(3S,4R)-4-[4-(3-chloro-2,4-difluoro-aniline)-7-methoxy-quinone Mixtures of oxazolin-6-yl]oxy-3-fluoro-piperidine-1-carboxylic acid butyl esters)
[0191] tert-butyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com