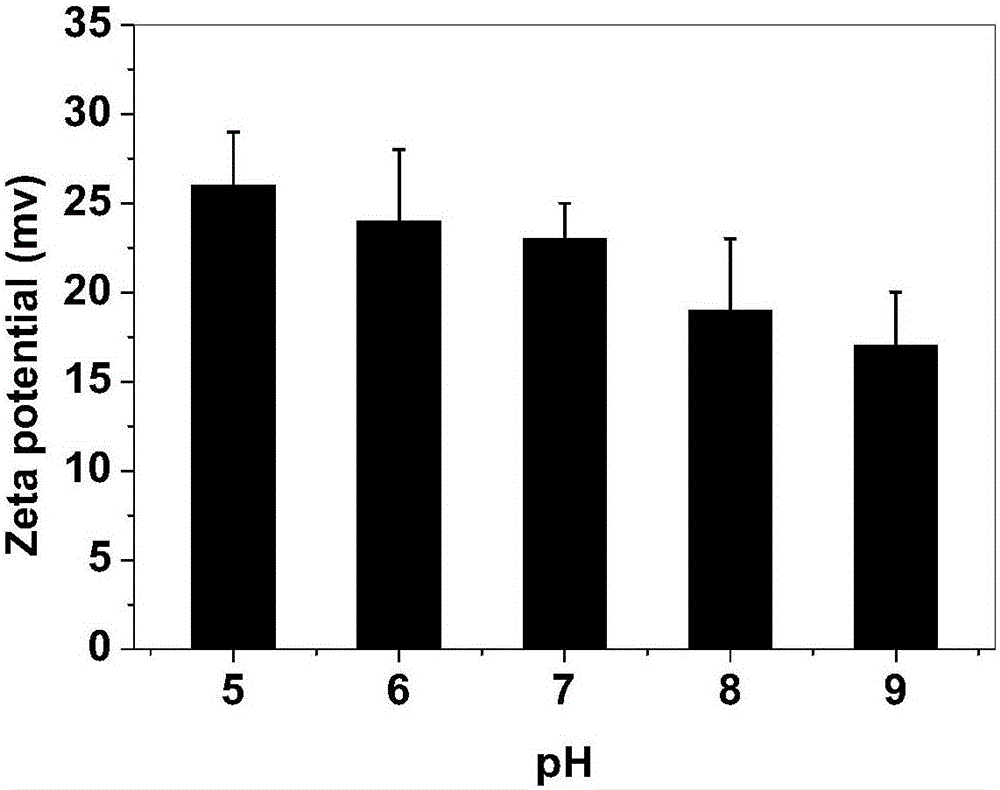
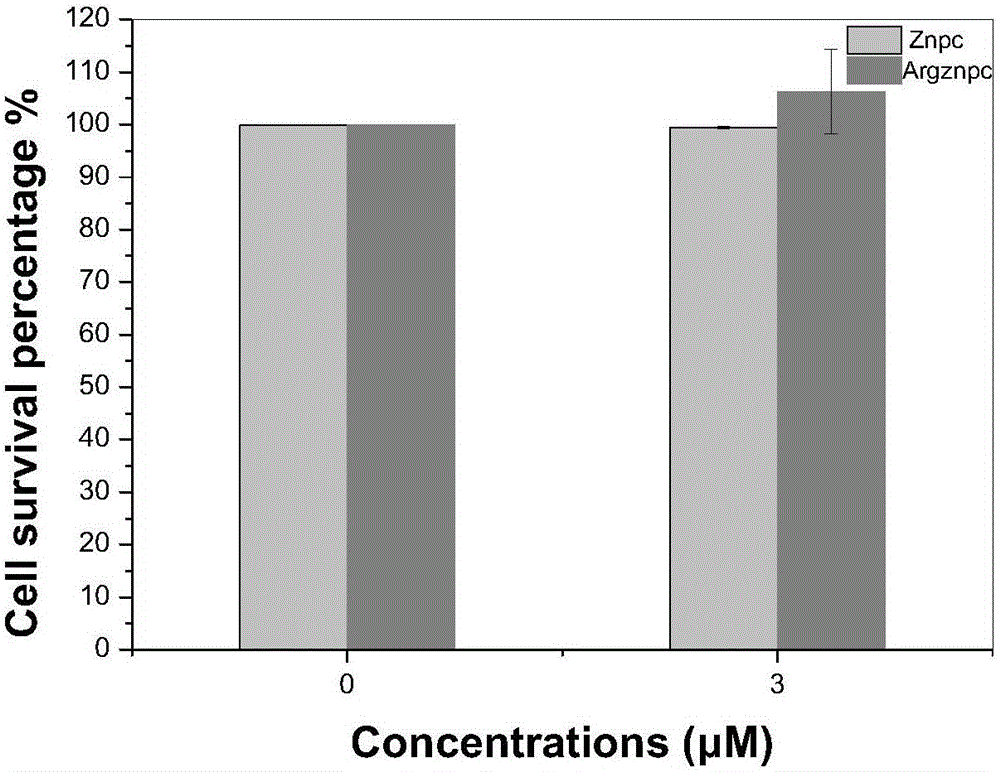
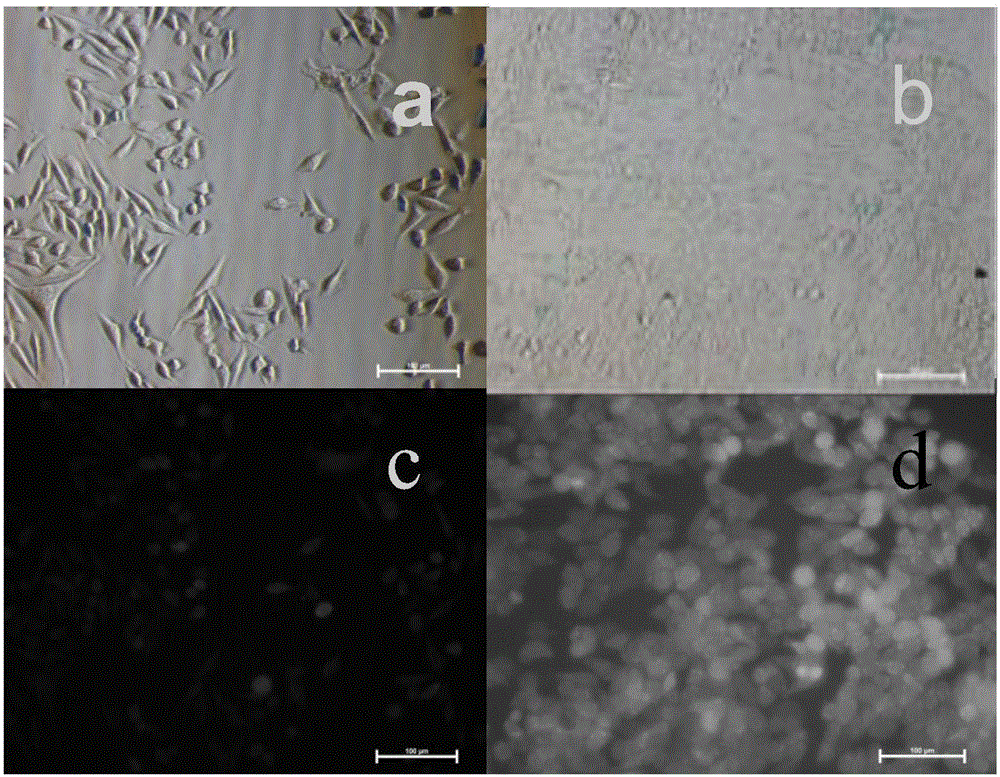
Arginine-substituted phthalocyanine derivatives, and synthesis method and application thereof
A technology of phthalocyanine derivatives and arginine, which is applied in the field of photosensitizers, can solve the problems of no targeting of tumor cells and no specific targeting of tumor tissues, and achieve inhibition of adhesion and proliferation, good application prospects, and dark toxicity low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) Under the protection of nitrogen, 4-nitrophthalonitrile, 4-hydroxybenzoic acid and basic catalyst are stirred and reacted at a molar ratio of 1:1:2, and DMF is used as a solvent, and the reaction is carried out at 40°C for 6 hours to obtain ortho Phthalonitrile intermediate 1;
[0043] (2) Under nitrogen protection, with n-amyl alcohol as solvent, Zn(OAc) 2 , The molar ratio of phthalonitrile intermediate 1 to DBU is 1:2:3; the reaction temperature is 120°C-140°C, the reaction time is 24h, the reaction solution is cooled to room temperature, and the solvent is distilled off under reduced pressure. The solid was dissolved with KOH aqueous solution, treated under reflux for 6 h and then cooled to room temperature. The filter cake was collected by filtration. The filter cake was dissolved with alkali again, and the insoluble part was removed by filtration. The pH of the filtrate was adjusted to be acidic to precipitate the product. The solid was collected after rep...
Embodiment 2
[0047] (1) Under the protection of nitrogen, 4-nitrophthalonitrile, 4-hydroxybenzoic acid and basic catalyst are stirred and reacted in a molar ratio of 1:3:4, and DMF is used as a solvent, and the reaction is carried out at 40°C for 6h to obtain ortho Phthalonitrile intermediate 1;
[0048] (2) Under the protection of nitrogen, with n-amyl alcohol as solvent, Cu(Ac) 2 , The molar ratio of phthalonitrile intermediate 1 to DBU is 1:2:4; the reaction temperature is 120°C-140°C, the reaction time is 24h, the reaction solution is cooled to room temperature, and the solvent is distilled off under reduced pressure. The solid was dissolved with KOH aqueous solution, treated under reflux for 6 h and then cooled to room temperature. The filter cake was collected by filtration. The filter cake was dissolved with alkali again, and the insoluble part was removed by filtration. The pH of the filtrate was adjusted to be acidic to precipitate the product. The solid was collected after re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com