Purpurin-18 ether derivatives and their preparation and use
A technology of purpurin and derivatives, applied in the field of medicine, can solve the problems of short tumor killing depth, small molar absorption coefficient, low photosensitivity, etc., and achieve excellent PDT killing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
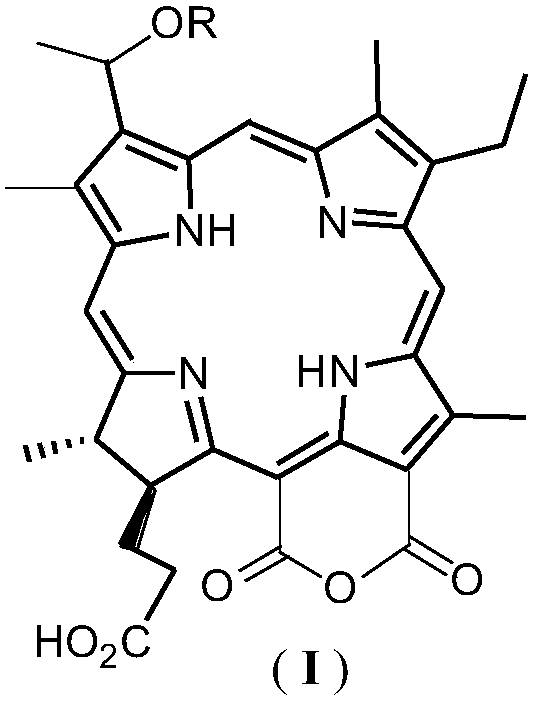
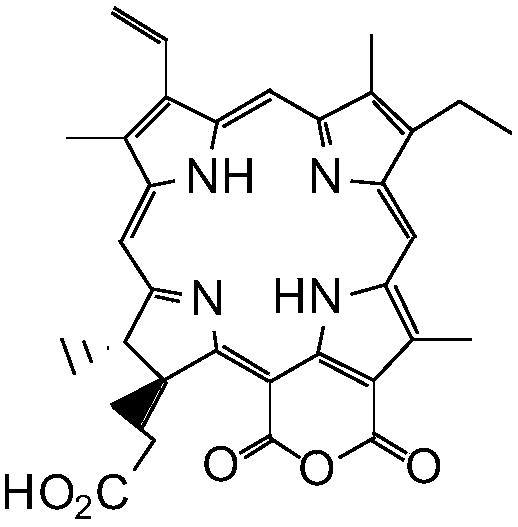
[0048] Embodiment 1: Preparation of pheophorbide a (IV)
[0049] The crude extract of chlorophyll a (Ⅴ) from silkworm excrement (paste chlorophyll) was purchased from Fengming Chlorophyll Co., Ltd., Haining City, Zhejiang Province.
[0050] Dissolve 100g of silkworm excrement chlorophyll a (Ⅴ) crude extract in 500mL ether, add an equal volume of concentrated hydrochloric acid at 0-5°C and stir for 1 hour, then separate the acid solution from the lower layer, add 2 times the amount of water to dilute, and use it under cooling 10mol / L NaOH neutralized to pH 5-6, suction filtered, P 2 o 5 After drying, 15 g of crude black powder IV was obtained, which was directly used in the next reaction without further purification.
Embodiment 2
[0051] Embodiment 2: the preparation of purpurin-18 (Ⅲ)
[0052] Ⅳ Dissolve 15g of crude product in 50mL of tetrahydrofuran, add 300mL of diethyl ether to dilute, then add 25% (w / v) potassium hydroxide n-propanol solution 20mL, and pass O at 0-5°C 2 Reacted for 2h, extracted with water (300mL×2), 10%H 2 SO 4 Neutralize, filter, P 2 o 5 It was dried and separated by silica gel H column chromatography to obtain 2.7 g of black powder III.
Embodiment 3
[0053] Example 3: Preparation of 3-(1-bromoethyl)-3-desvinylpurpurin-18(Ⅲ)
[0054] Compound III (1.0 g) was added with 100 mL of 33% HBr glacial acetic acid solution, sealed at room temperature for 24 hours, and the glacial acetic acid was evaporated under reduced pressure to obtain dark green solid compound II, which was directly used in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com