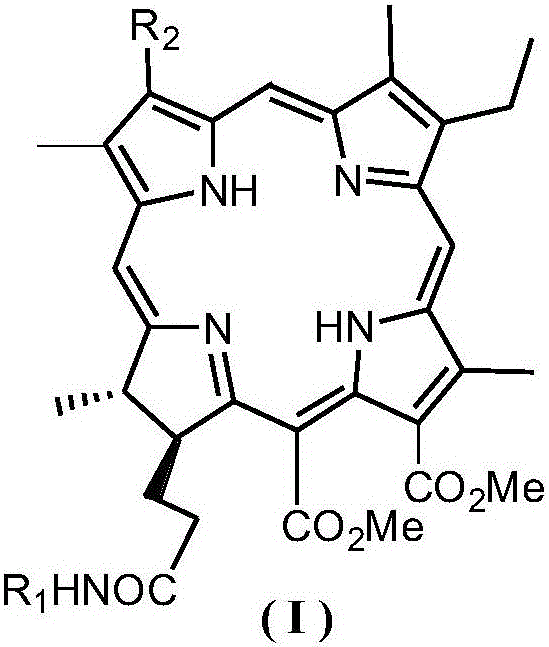
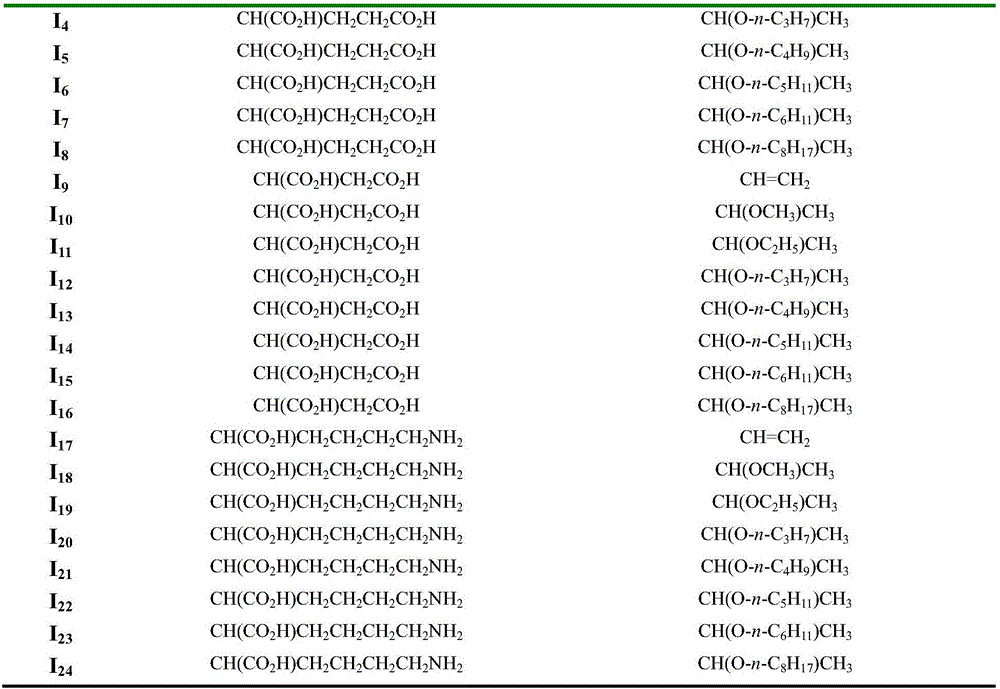
Chlorin p6 amino acid derivative, preparation method therefor and use of chlorin p6 amino acid derivative
A chlorin and amino acid technology, applied in the field of medicine, can solve the problems of short tumor killing depth, low photosensitivity activity, slow clearance, etc., and achieve the effect of excellent PDT killing effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: Preparation of pheophorbide a (VII)
[0055] Chlorophyll a (Ⅷ) crude extract from silkworm excrement (paste chlorophyll) was purchased from Fengming Chlorophyll Co., Ltd., Haining City, Zhejiang Province.
[0056] Dissolve 100g of silkworm excrement chlorophyll a (Ⅷ) crude extract in 500mL ether, add an equal volume of concentrated hydrochloric acid at 0-5°C and stir for 1 hour, take the lower layer of acid solution, add 2 times the amount of water to dilute, and use it under cooling 10mol / L NaOH neutralized to pH 5-6, suction filtered, P 2 o 5 After drying, 15 g of crude black powder VII was obtained, which was directly used in the next reaction without further purification.
Embodiment 2
[0057] Embodiment 2: Preparation of purpurin-18 (Ⅵ)
[0058] VII Crude product 15g, dissolved in 50mL tetrahydrofuran, added 300mL diethyl ether to dilute, then added 25% (w / v) potassium hydroxide n-propanol solution 20mL, passed through O at 0-5°C 2 Reacted for 2h, extracted with water (300mL×2), 10%H 2 SO 4 Neutralize, filter, P 2 o 5 It was dried and separated by silica gel H column chromatography to obtain 2.7 g of black powder VI.
Embodiment 3
[0059] Example 3: Chlorin p 6 Preparation of Trimethyl Ester (Ⅴ)
[0060] Compound VI (2.0g, 3.55mmol), dissolved in 50mL of tetrahydrofuran, added 200mL of methanol, and 250mL of 0.5mol L-1 sodium hydroxide aqueous solution, stirred at room temperature until the absorption peak at 698nm disappeared, added 500ml of water, and dissolved in dilute hydrochloric acid and to pH 5-6, extracted with ether (3×200mL), dried over anhydrous sodium sulfate, filtered, added freshly prepared 2.8% (w / v) CH 2 N 2 Diethyl ether solution 20mL, reacted for 5min, washed with water, recovered solvent, dried and separated by silica gel H column chromatography to obtain 1.85g black powder Ⅴ, yield 83.6%, purity (HPLC): 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com