Tumor vascular endothelial cell marker 8 mutant, its fusion protein and application
A technology of endothelial cells and fusion proteins, applied in the field of molecular biology, can solve the problem of no progress in TEM8 structural research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
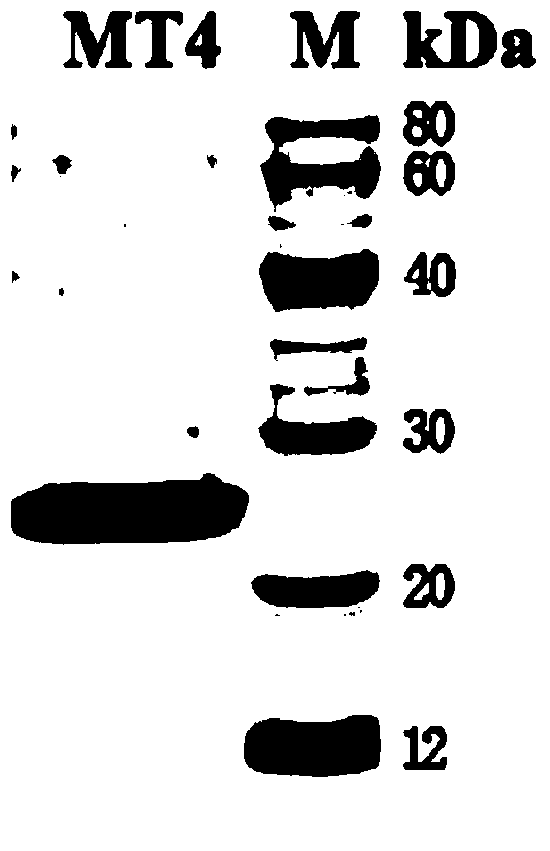
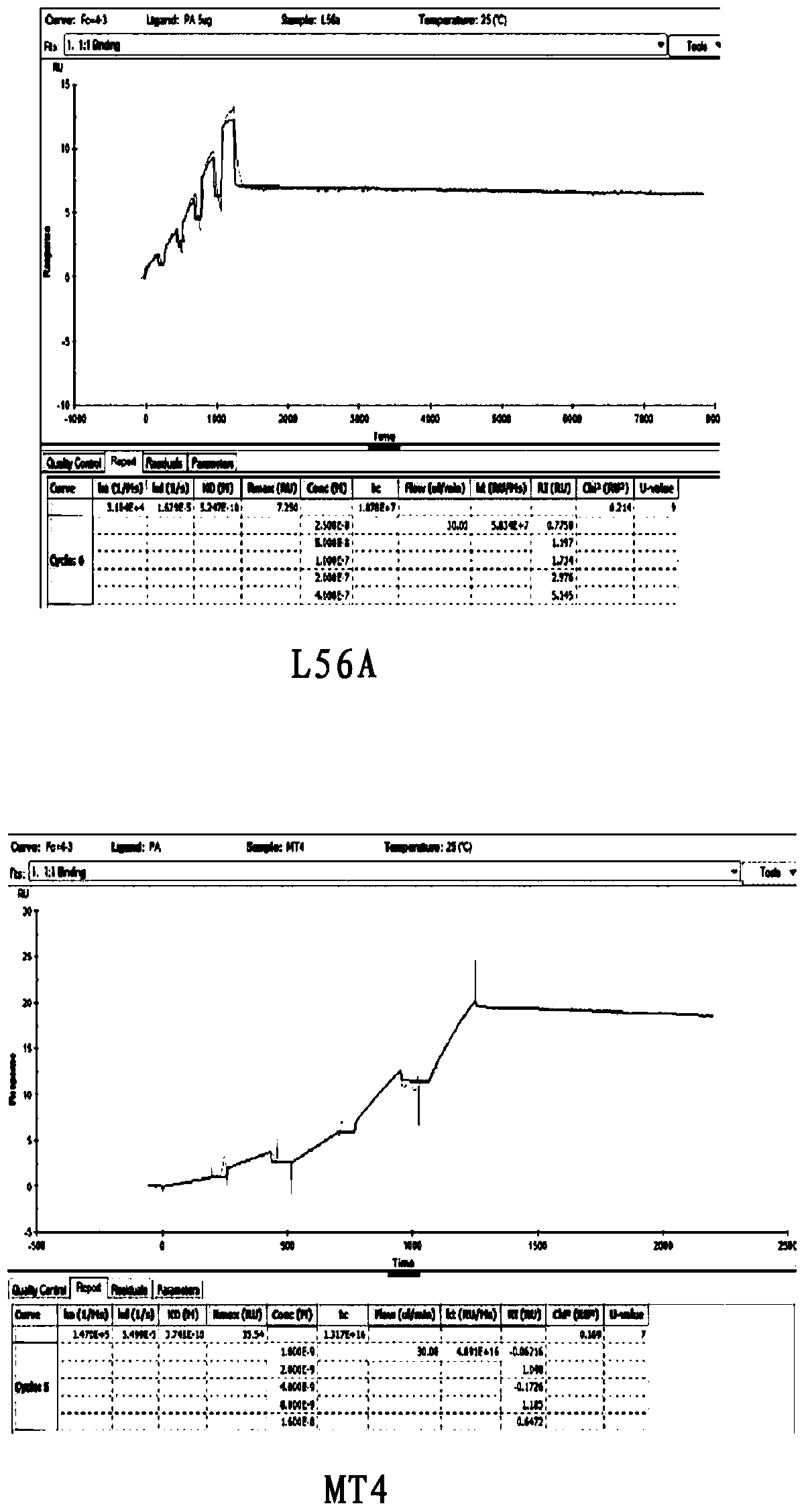
[0035] Example 1 Expression of TEM8 mutant MT4
[0036] 1. Expression plasmid construction of mutant MT4
[0037] Using L56A-PHAT2 as a template, mutate the 152, 154-159 amino acids by inverse PCR technology, the reaction system is 50 μL, see Table 1 for details.
[0038] Table 1. PCR reaction system (1)
[0039]
[0040] The reaction conditions are:
[0041]
[0042] The PCR product was digested as a template, then phosphorylated and ligated overnight; transformed into DH5α competent cells, spread on LB plates containing Amp, and cultured until clonal growth.
[0043] Primer sequence:
[0044] MT4-R: GCCATCGAGTTTTTCCATCAGTCAAAGCAATGATG
[0045] MT4-F: CTGGTGCCGAGCTATTCAGAGAGGGAGGCTAATAG
[0046] 2. Protein expression and purification
[0047] Transform the MT4-PHAT2 plasmid that has been sequenced and confirmed to have the correct sequence into BL21 Escherichia coli competent, smear the plate and wait for the clones to grow out, pick a single clone and culture it ...
Embodiment 2
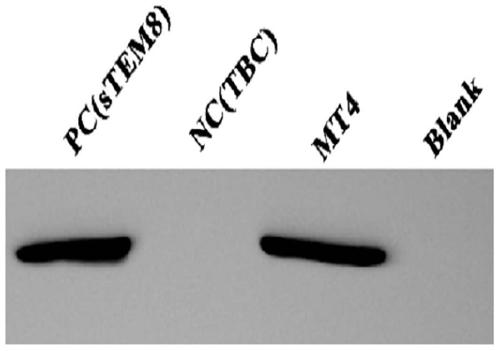
[0067] Example 2 Expression and analysis of HAS-MT4 fusion protein
[0068] 1. Construction of HSA and MT4 fusion protein
[0069] Fusion with HSA is a commonly used method to extend protein half-life. In order to construct the HSA-MT4 fusion protein more conveniently, we modified the existing yeast expression plasmid pMEX9K-HSA-CMG2, and inserted two unique restriction enzyme sites of NgoMIV and SpeI between the linker and CMG2, Use one of the restriction enzyme sites and the existing NotI on the plasmid to connect MT4 into the expression vector, as shown in the schematic diagram Image 6 shown.
[0070] The PCR process includes two steps: the first step is to add NgoMIV / SpeI and NotI restriction sites at both ends of MT4. The reaction system was 50 μL, see Table 2 for details.
[0071] Table 2. PCR reaction system (2)
[0072]
[0073] The reaction conditions are:
[0074]
[0075] The second step of PCR is to add the NgoMIV / SpeI restriction site after the HSA-CM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com