A kind of purification method of ethyl 7-chloro-2-oxoheptanoate
A technology of ethyl oxoheptanoate and purification method, applied in chemical instruments and methods, preparation of carboxylate, organic chemistry, etc., can solve problems such as multiple impurities in ethyl 7-chloro-2-oxoheptanoate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
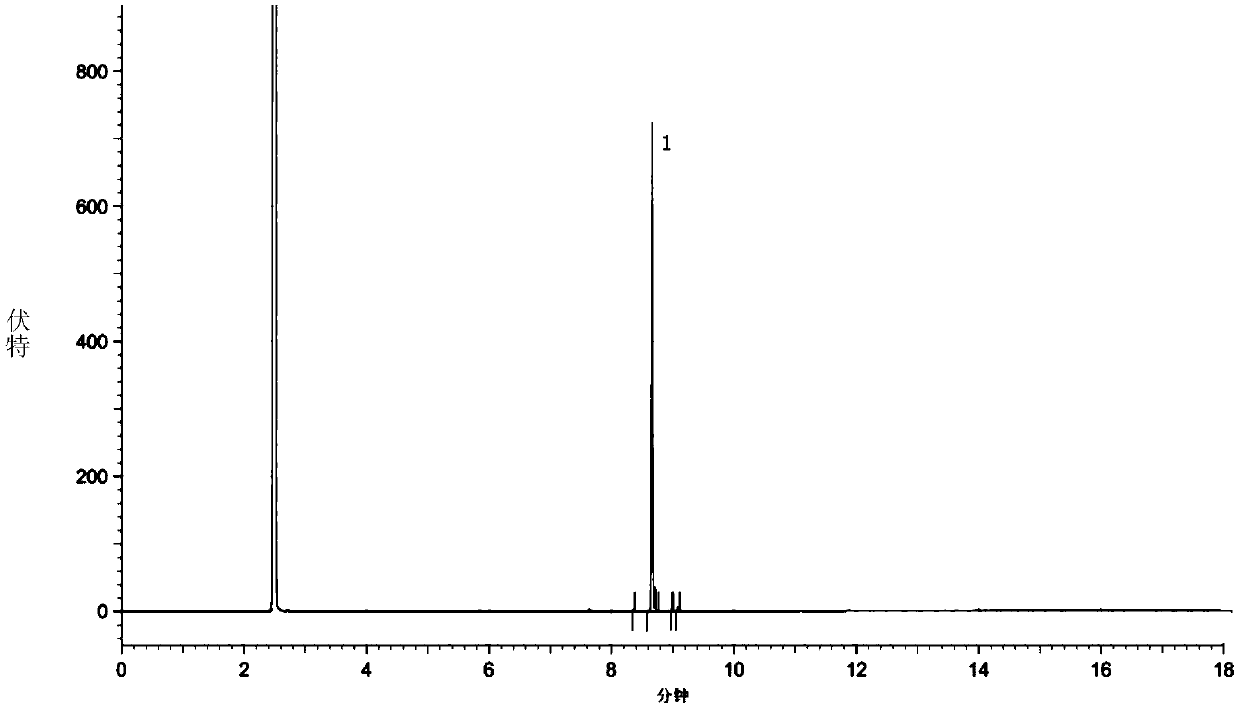
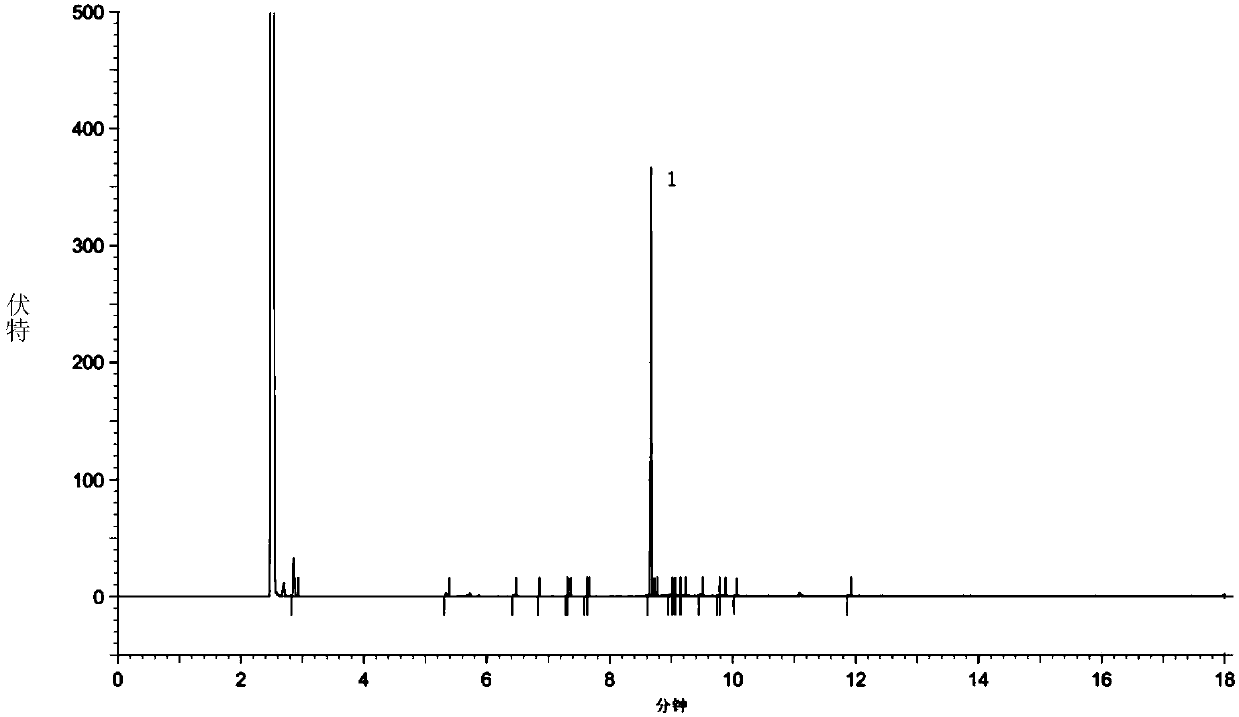
Image
Examples
Embodiment 1
[0083] Example 1 Purification of ethyl 7-chloro-2-oxoheptanoate
[0084] 1) At room temperature, 50.0 g of crude ethyl 7-chloro-2-oxoheptanoate (oil, GC normalized content 60.6%, external standard content 58.3%, equivalent to containing 7-chloro-2-oxoheptanoate Ethyl ester (29.2 g, 0.14 mol) was slowly dropped into a saturated solution prepared by dissolving 21.9 g of sodium bisulfite (0.21 mol) in 37 ml of water, and the rate of addition was controlled to maintain the temperature of the reaction solution at room temperature. After the dropwise addition, stir at room temperature, and a white solid precipitates out after about 0.5 h.
[0085] 2) The reaction mixture obtained in step 1) was continuously stirred at 0-5°C for 2 hours, then filtered, and the filter cake was washed with an appropriate amount of absolute ethanol, drained, and dried to obtain ethylene oxide of ethyl 7-chloro-2-oxoheptanoate Sodium hydrogen sulfate (white solid) 40.1g.
[0086] 3) At room temperat...
Embodiment 2
[0090] Example 2 Purification of ethyl 7-chloro-2-oxoheptanoate
[0091] 1) At room temperature, dissolve 18.7g of sodium bisulfite (0.18mol) in 32ml of water, then add 11ml of absolute ethanol and stir well; 50.0g of crude ethyl 7-chloro-2-oxoheptanoate (oil , GC normalized content of 64.3%, external standard content of 62.4%, which is equivalent to containing 31.2 g of 7-chloro-2-oxoheptanoic acid ethyl ester, 0.15 mol) slowly dropwise to keep the temperature of the reaction solution at room temperature. After the dropwise addition, stir at room temperature, and a white solid precipitates out after about 0.5 h.
[0092] 2) The reaction mixture obtained in step 1) was continuously stirred at 0-5°C for 3 hours, then filtered, and the filter cake was washed with an appropriate amount of ethyl acetate, sucked dry, and dried to obtain ethylene oxide of ethyl 7-chloro-2-oxoheptanoate Sodium bisulfate salt (white solid) 43.9g.
[0093] 3) At room temperature, 43.9 g of the whi...
Embodiment 3
[0095] Example 3 Purification of ethyl 7-chloro-2-oxoheptanoate
[0096] 1) At room temperature, 50.0 g of crude ethyl 7-chloro-2-oxoheptanoate (oil, GC normalized content 50.8%, external standard content 49.6%, equivalent to containing 7-chloro-2-oxoheptanoate Ethyl acetate (24.8g, 0.12mol) was dissolved in 50ml of absolute ethanol, then slowly dripped into a saturated solution made of 16.2g sodium bisulfite (0.16mol) dissolved in 28ml of water, and the rate of addition was controlled so that the temperature of the reaction solution Maintain room temperature. After the dropwise addition, stir at room temperature, and a white solid precipitates out after about 0.5 h.
[0097] 2) The reaction mixture obtained in step 1) was continuously stirred at 0-5°C for 2 hours, then filtered, and the filter cake was washed with an appropriate amount of toluene, drained, and dried to obtain hydrogen sulfite of ethyl 7-chloro-2-oxoheptanoate Sodium (white solid) 34.7g.
[0098] 3) At r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com