Synthetic method of 5-hydroxymethyl furfural doped 2,3-unsaturated glucoside
A technology for the synthesis of hydroxymethylfurfural and a synthesis method, which is applied in the field of synthesis of 2,3-unsaturated glycosides, can solve the problems of low synthesis efficiency, catalyst use efficiency, catalyst inability to recycle, and inability to recycle, etc., to achieve low production cost, The effect of convenient operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
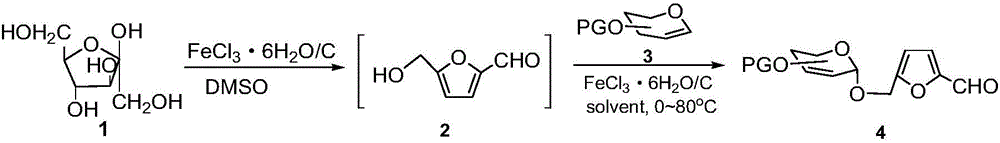
[0017] 216.8mg (0.80mmoL) of D-fructose and 2ml of dimethyl sulfoxide in 55mg of FeCl 3 ·6H 2 Under the catalysis of O / C (0.08mmoL) solid acid, the Ferrier rearrangement reaction was carried out, and the synthesized 5-hydroxymethylfurfural 101.3mg (0.80mmol) was directly used as the acceptor and donor in the next Ferrier rearrangement reaction without separation. After mixing 182.2mg (0.67mmol) of fully acetylated glucosene, add 7ml of dichloromethane and stir well, carry out Ferrier rearrangement reaction at room temperature, TLC (PE:EA=1:1) monitors the reaction is complete, and the catalyst is filtered out , dried and recovered for later use, the filtrate was concentrated and purified to obtain 185.3 mg of 2,3-unsaturated-4,6-di-O-acetylglucose 5'-furfuralmethanol glycoside, with a yield of 85%.
[0018] After nuclear magnetic spectrum detection and mass spectrometry analysis of the product obtained in Example 1, it can be determined that its structure is the target produc...
Embodiment 2
[0021] After mixing 99mg (0.77mmol) of 5-hydroxymethylfurfural with 81.6mg (0.63mmol) of fully acetylated glucosene, add 6ml of dichloromethane and stir thoroughly, and add 43mg of the first-time recycling in Example 1 at room temperature FeCl 3 ·6H 2 O / C solid acid catalyst (0.063mmoL) carries out Ferrier rearrangement reaction, TLC (PE:EA=1:1) monitoring reaction is complete, the catalyst is filtered out, dried and recovered for later use, and the filtrate is concentrated and purified to obtain the product as 2,3-unsaturated-4,6-di-O-acetylglucose 5'-furfural methanoside 160 mg, the yield was 76.5%.
Embodiment 3
[0023] After mixing 100mg (0.70mmol) of 5-hydroxymethylfurfural with 73.5mg (0.57mmol) of fully acetylated glucosene, add 5ml of dichloromethane and stir thoroughly, and add 37mg of the second recovery in Example 2 at room temperature FeCl 3 ·6H 2O / C solid acid catalyst (0.056mmoL) carries out Ferrier rearrangement reaction, TLC (PE:EA=1:1) monitoring reaction is complete, the catalyst is filtered out, dried and recovered for later use, and the filtrate is concentrated and purified to obtain the product as 2,3-unsaturated-4,6-di-O-acetylglucose 5'-furfural methanoside 126 mg, the yield was 66%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com