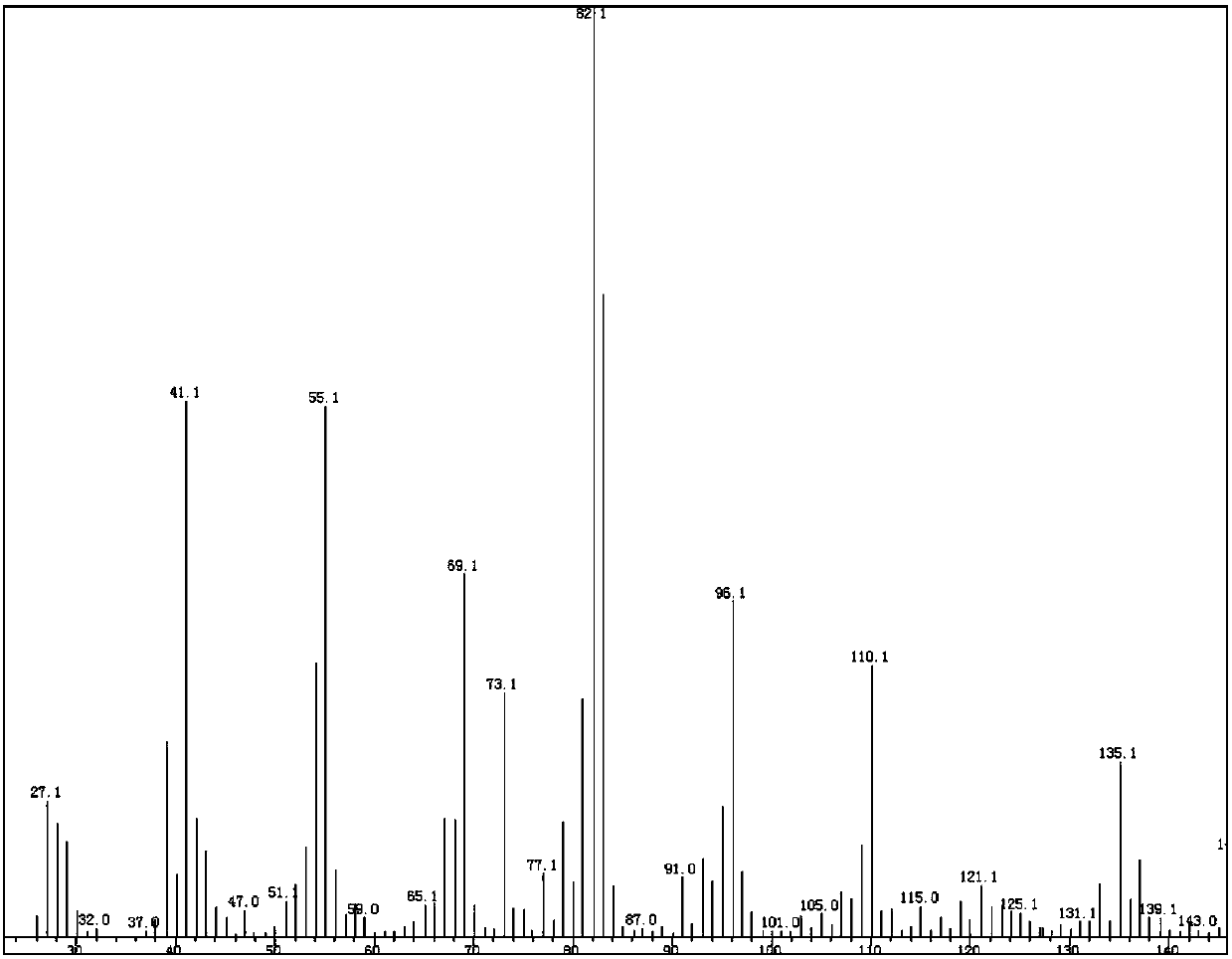
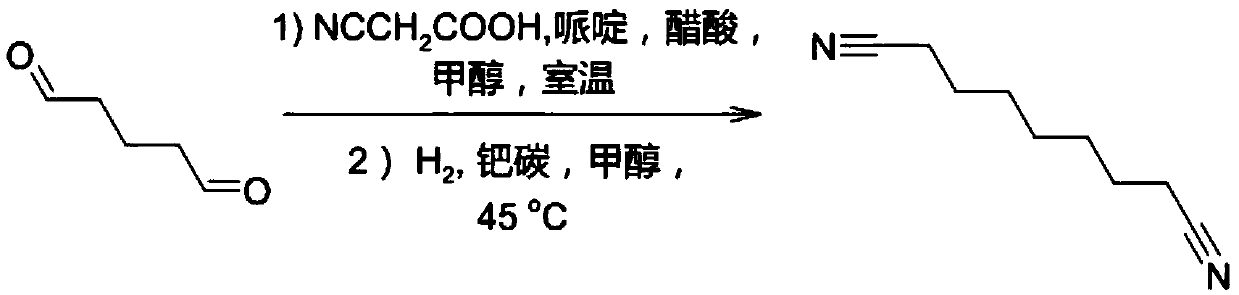
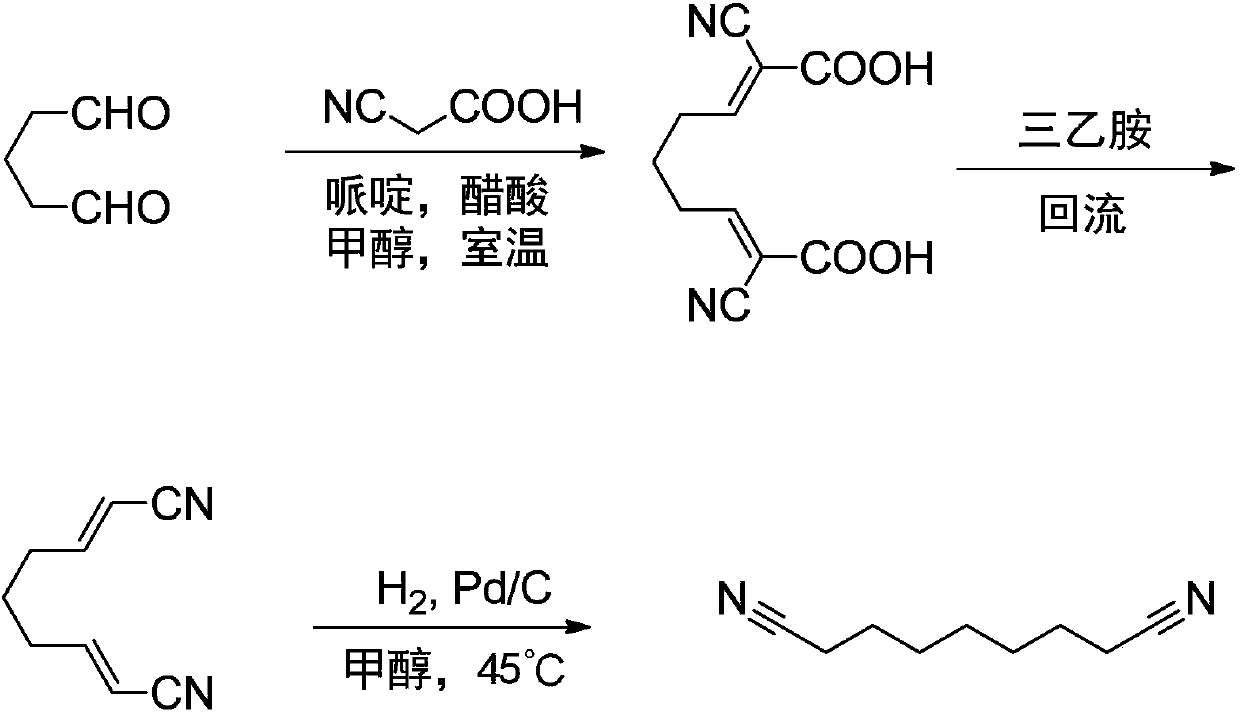
Azelanitrile preparation method
A technology for azelaic nitrile and glutaraldehyde, which is applied in the field of preparing azelaic nitrile by one-pot method, can solve the problems of intractable treatment of three wastes, high cost of raw materials, corrosiveness, etc., and achieves low cost, reduced production cost and good yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Using methanol as solvent to prepare azelaonitrile
[0046] The reaction formula is as follows:
[0047]
[0048] (1) Condensation, decarboxylation and hydrogenation reactions: In an autoclave, add 50g (0.5mol) glutaraldehyde, 250g methanol, stir to dissolve, at room temperature, add 2.5g (29.4mmol) piperidine, 1.77g (29.5mmol) ) Acetic acid, the mixture is stirred for 60 minutes, and then 85 g (1 mol) of cyanoacetic acid is added in batches. During the addition, the temperature of the system is maintained not to exceed 40°C. After the addition is complete, continue to stir at room temperature for 2 to 3 hrs. Subsequently, in the autoclave, continue to add 5g palladium-carbon catalyst (5% water), seal the reactor, evacuate, replace with nitrogen, and then pass in hydrogen, the pressure is maintained at 4.0MPa, the reaction temperature is maintained at 45°C, the reaction continues During 12hrs, every 2hrs, use the purge valve to release the carbon dioxide generated by the r...
Embodiment 2
[0051] Using ethanol as solvent to prepare azelaonitrile
[0052] Azelaonitrile was prepared in the same manner as in Example 1, except that 250 g of ethanol was used instead of 250 g of methanol in step (1). The resulting azelaonitrile yield was 70.1% and purity was 98.1%.
Embodiment 3
[0054] Preparation of Azela Nitrile with N,N-Dimethylformamide as Solvent
[0055] Except that 250 g of N,N-dimethylformamide was used instead of 250 g of methanol in step (1), the reaction was performed in the same manner as in step (1) of Example 1.
[0056] Then the obtained reaction liquid is subjected to vacuum distillation, the temperature is controlled not to be higher than 100° C. The solvent N,N-dimethylformamide is distilled out, and then the temperature is raised for vacuum distillation to collect azelaonitrile products.
[0057] The obtained azela nitrile yield was 77.8%, and the purity was 98.9%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com