MAO inhibitors and their conjugates as therapeutics for the treatment of brain cancer
An inhibitor, brain cancer technology, applied in medical preparations containing active ingredients, drug combinations, organic active ingredients, etc., can solve problems such as limited treatment options
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
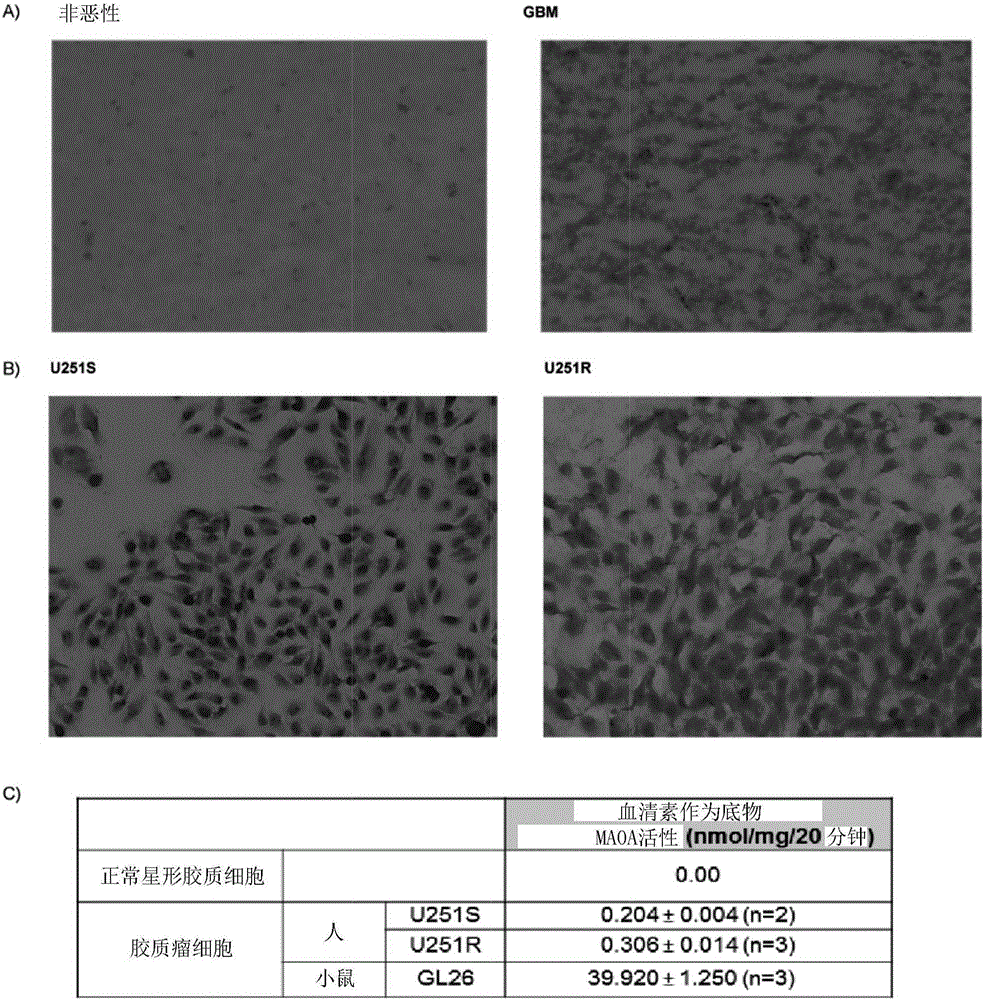
[0172] Expression of MAO A in human glioma tissues and cells
[0173] The results showed that there was prominent staining of MAO A in GBM tissues, while no detectable staining was observed in non-tumor brain tissues ( figure 2 A). Based on morphology, the staining of GBM appeared to be associated with tumor cells. As shown by immunostaining ( figure 2 B), Both sensitive (U251S) and resistant (U251R) human glioma cells express MAO A. Then using serotonin as a substrate ( figure 2 C) These glioma cell cultures were analyzed for MAO activity (5) and were shown to express MAO A catalytic activity; in contrast, normal control astrocytes exhibited no detectable MAO A activity.
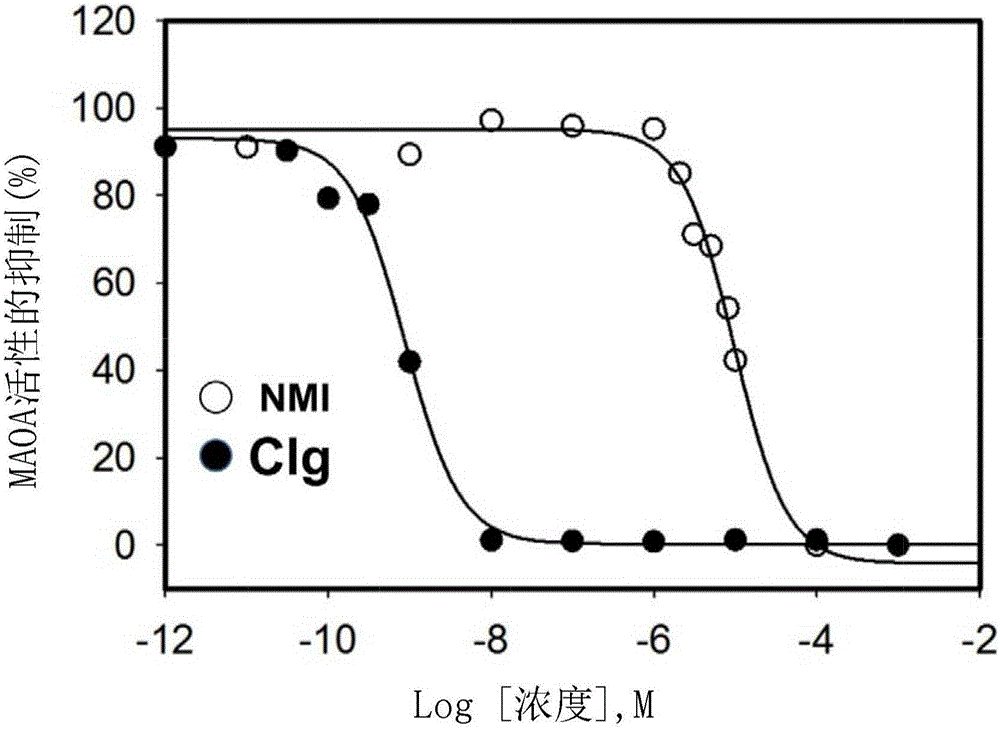
[0174] MAO A activity was also measured in the mouse glioma cell line GL26; these tumor cells showed high levels of MAO A activity. Based on this information, GL26 cells were chosen to evaluate the inhibitory effect of different MAO inhibitors. Clogiline and phenelzine inhibit MAO A activity with 1...
Embodiment II
[0176] MAO A inhibitors clogiline and NMI (near-infrared dye-conjugated MAO inhibitor) reduce proliferation, viability and migration of TMZ-sensitive and resistant glioma cells
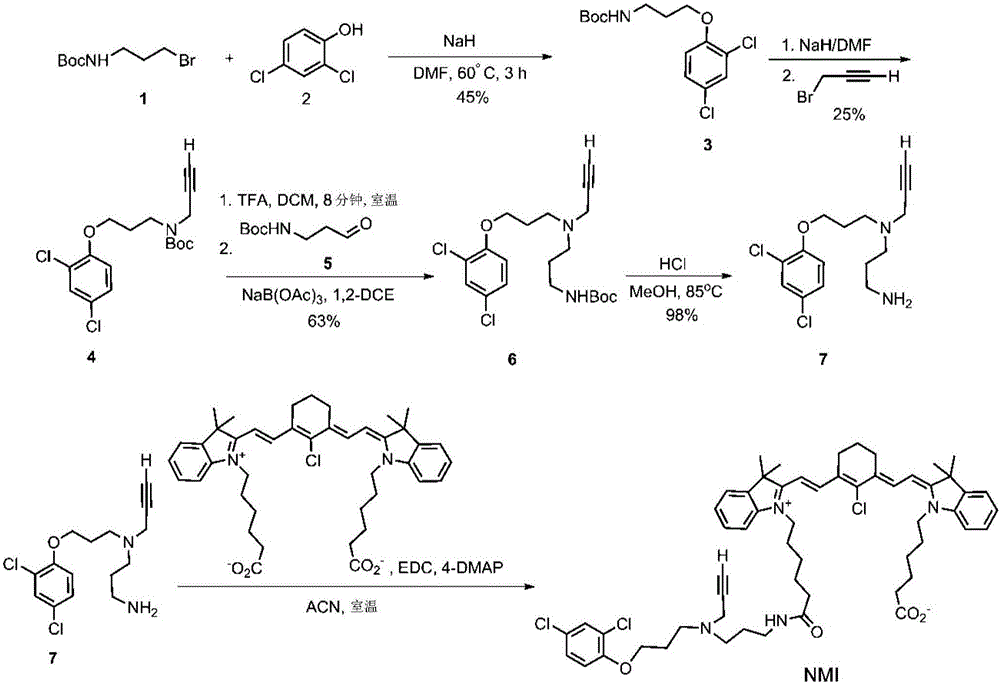
[0177] MAO A inhibitors target MAO A expression in both the central nervous system and peripheral tissues. To specifically target MAOA to brain cancer cells, the MAO inhibitor clogiline was conjugated to the near-infrared (NIR) tumor-specific dye MHI-148 to generate a novel drug NMI ( Figure 5 A), It accumulates preferentially in cancerous lesions. NMI has been based on figure 1 Synthesized by the steps shown in.
[0178]To evaluate the cellular uptake of NMI in human glioma cells, confocal microscopy was used. Image analysis of tumor cells treated with NMI (1 μM, 5 μM) showed significant dose-dependent uptake compared to vehicle. As determined by the mitochondria-specific dye MitoTracker Green ( Figure 5 B), The compound rapidly accumulates and localizes to mitochondria in U251R cells. Inhibi...
Embodiment III
[0185] Genetically modified MAO A knockout (MAO A KO) animals exhibit increased survival.
[0186] IHC was used to determine the expression of MAO A in human GBM tissues ( figure 2 B), In contrast less staining of MAO A in non-tumor brain. These results showed that the higher expression of MAO A in glioma cells and GBM tissues indicated that MAO A was involved in GBM progression.
[0187] To examine the in vivo effect of MAO A on tumor growth, tumor progression was analyzed in MAO A knockout mice (KO) in C57bl / 6 mice. Mouse glioma cells (GL26) derived from C57bl / 6 mice were used in these studies because these tumor cells showed high levels of MAO A activity ( figure 2 C). Tumor cells were luciferase-labeled and implanted intracranially in MAO AKO and WT C57bl / 6 mice; luciferase imaging was performed on day 10. The results showed a 75% reduction in tumor burden ( Figure 6 A and B), survival increased by 17.6% (3 days) ( Figure 6 C).
[0188] MAO A activity was determ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com