Application of astragalus extract
A technology of astragalus extract and astragalus, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The preparation of embodiment 1 Astragalus extract
[0064] (1) extraction
[0065] Astragalus decoction pieces 50kg, reflux with 250L of 50% aqueous ethanol to obtain the extract according to the conventional method, filter the extract after concentrating under reduced pressure, and take the filtrate for subsequent use;
[0066] (2) Purification
[0067] The above-mentioned filtrate was subjected to D101 macroporous adsorption resin adsorption, and after removing impurities by water elution, it was eluted with 60% aqueous ethanol, and the 60% ethanol eluate was collected;
[0068] (3) concentrated drying
[0069] The eluate was concentrated under reduced pressure and dried to obtain 8.5 kg of Astragalus extract;
[0070] (4) Content determination
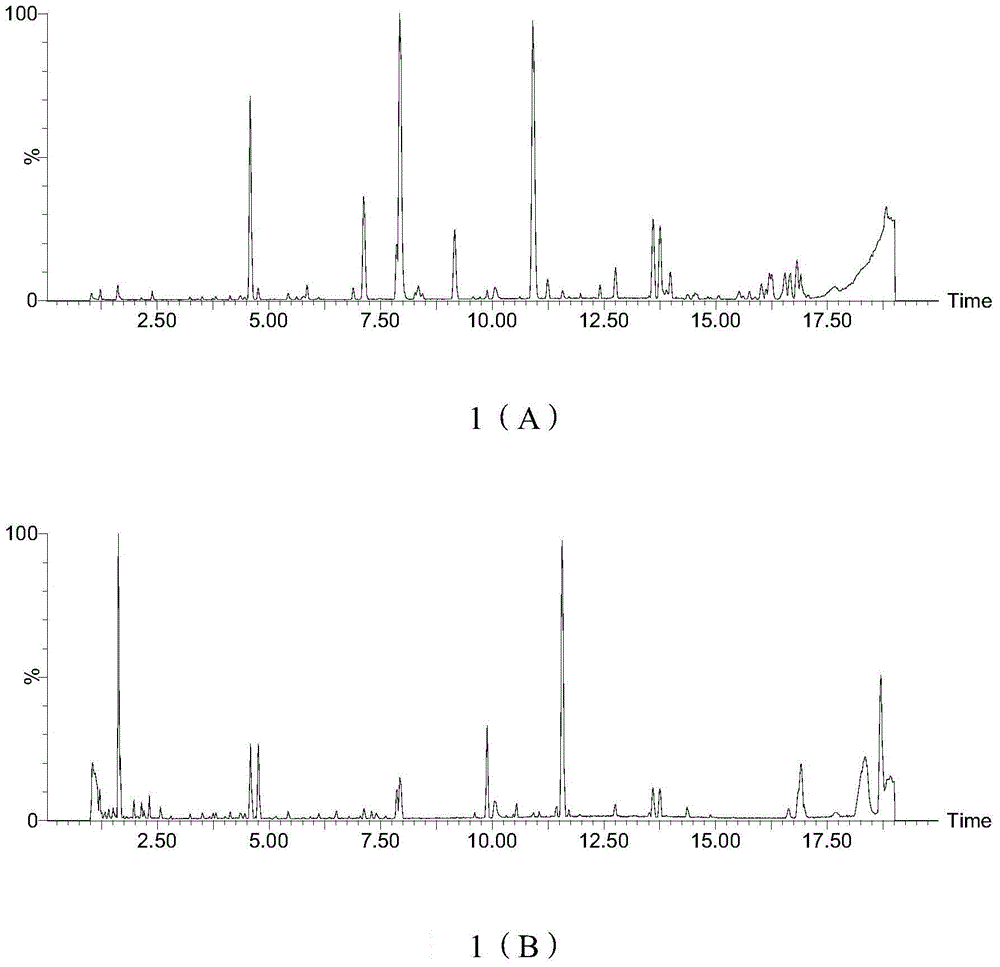
[0071] Astragaloside IV Chromatographic condition C 18(4.6*250mm 5μm); acetonitrile water (32:68) as mobile phase, flow rate 1ml / min, detected by evaporative light scattering detector, flow rate 1.60L / min; evaporation t...
Embodiment 2
[0080] The preparation of embodiment 2 Astragalus extract
[0081] (1) extraction
[0082] Astragalus decoction pieces 50kg, 300L of 70% ethanol to obtain the extract according to the conventional reflux method, the extract is decompressed to recover ethanol, filtered after concentration, and the filtrate is taken for subsequent use;
[0083] (2) Purification
[0084] The above-mentioned filtrate is subjected to AB8 macroporous adsorption resin adsorption, and after removing impurities by water elution, it is eluted with 50% aqueous ethanol, and the 50% ethanol eluate is collected;
[0085] (3) concentrated drying
[0086] The eluate was concentrated under reduced pressure and dried to obtain 7.2 kg of Radix Astragali extract;
[0087] (4) Content determination
[0088] Referring to the content determination method under the item of Example 1, the content of astragaloside IV in the Radix Astragali extract was measured to be 0.92%, and the content of calycosin glucoside was...
Embodiment 3
[0089] The preparation of embodiment 3 Astragalus extract
[0090] (1) extraction
[0091] Astragalus decoction pieces 50kg, reflux with 250L of 30% aqueous ethanol according to conventional methods to obtain the extract, the extract is concentrated under reduced pressure to a relative density of 0.85 at 25°C, filtered, and the filtrate is taken for subsequent use;
[0092] (2) Purification
[0093] The above-mentioned filtrate is subjected to D101 macroporous adsorption resin adsorption, and after removing impurities by water elution, it is eluted with 10% aqueous ethanol, and the 10% ethanol eluate is collected;
[0094] (3) concentrated drying
[0095] The eluate was concentrated under reduced pressure and dried to obtain 8.5 kg of Astragalus extract;
[0096] (4) Content determination
[0097] Astragaloside IV Chromatographic condition C 18 (4.6*250mm 5μm); acetonitrile water (32:68) as mobile phase, flow rate 1ml / min, detected by evaporative light scattering detector...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com