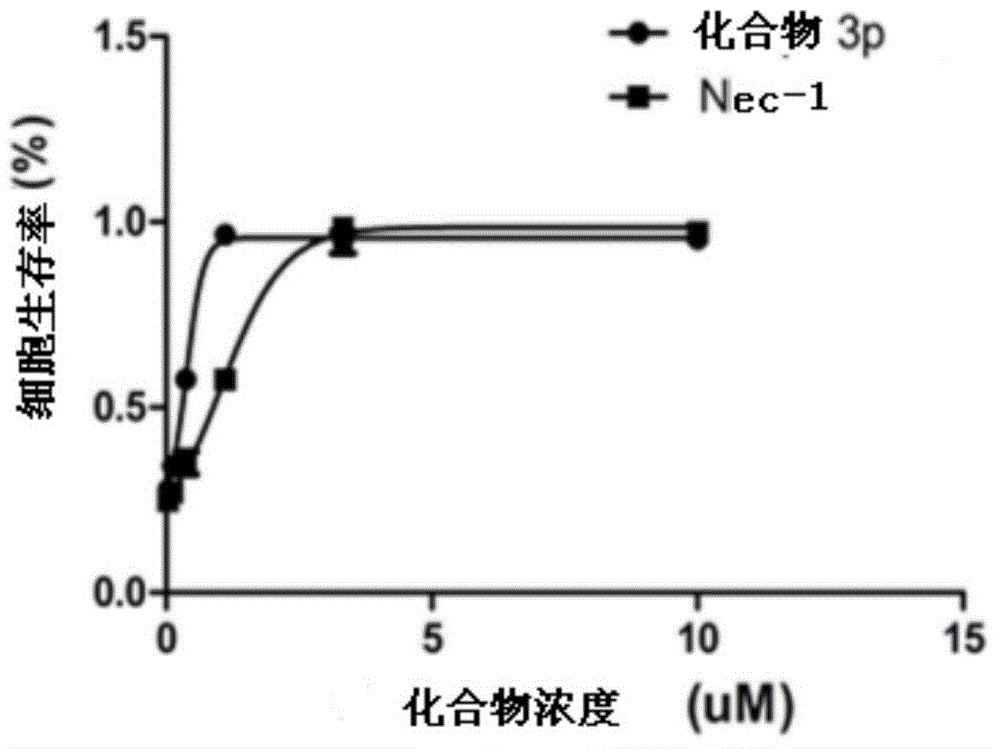
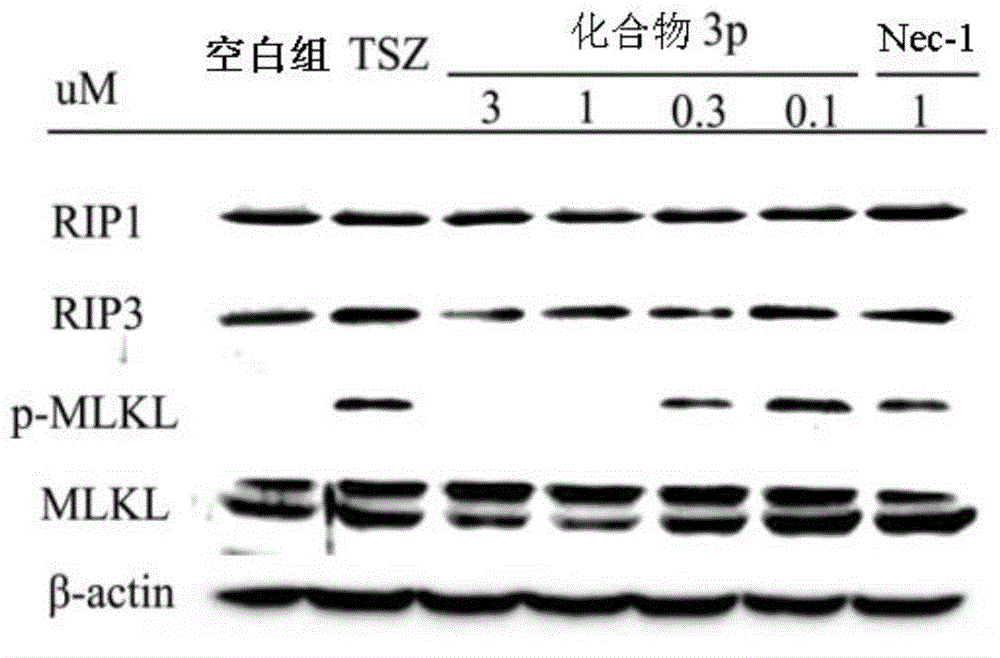
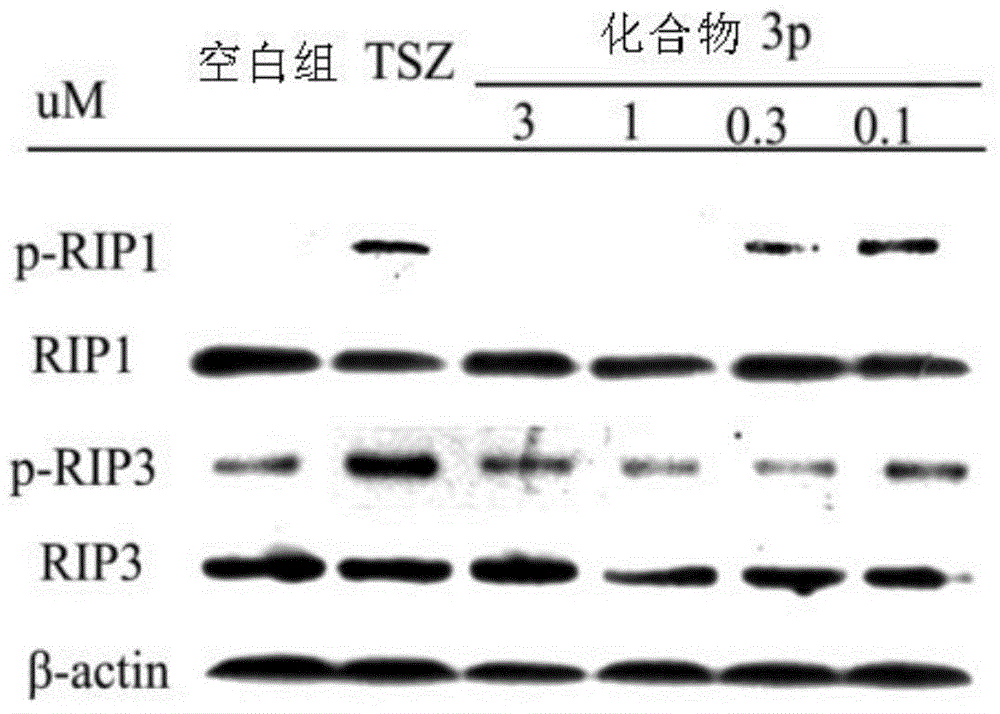
Benzyl-1H-pyrazol and benzyl-1H-pyrrole derivative and preparation method and application thereof
A technology of pyrrole derivatives and benzyl, which is applied in the field of chemical medicine and can solve problems such as poor pharmacokinetic properties, limited research, and poor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 Preparation of 4-chloro-3-nitro-1H-pyrazole (intermediate 2a)
[0062]
[0063] Add 3-nitro-1H-pyrazole (5mmol) and NBS (5.5mmol) to the reaction flask, add 20mL DMF (N,N-dimethylformamide), then heat to 90°C and react overnight under nitrogen protection . After the reaction was complete, most of the DMF was distilled off under reduced pressure, and a large amount of solid was precipitated after adding water, filtered, washed with 20 mL of water, and dried to obtain the target product as a light yellow solid. (yield 87%)
[0064] 1 H-NMR (400MHz, DMSO-d 6 )δ:14.32(s,1H),8.37(s,1H); MS(ESI),m / z:148.1[M+H] + . Intermediates 2b, 2c were prepared by the same method as above.
Embodiment 2
[0065] Example 2 Preparation of 4-bromo-3-nitro-1H-pyrazole (intermediate 2b)
[0066]
[0067] Using the same method as the preparation of intermediate 2a, intermediate 2b was prepared with a yield of 55%.
[0068] 1 H-NMR (400MHz, DMSO-d 6 )δ:14.38(s,1H),8.45(s,1H); m / z:192.2[M+H] + .
Embodiment 3
[0069] Example 3 Preparation of 4-iodo-3-nitro-1H-pyrazole (intermediate 2c)
[0070]
[0071] Using the same method as the preparation of intermediate 2a, intermediate 2c was prepared with a yield of 20%.
[0072] 1 H-NMR (400MHz, DMSO-d 6 )δ:14.31(s,1H),8.27(s,1H); MS(ESI),m / z:148.1[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com