Pharmaceutical applications of poly-cationic resin
A polycationic resin and drug technology, applied in the field of medicine, can solve the problem of no treatment method and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Embodiment 1, the establishment of metabolic syndrome disease model and the control of body weight by polystyrene quaternary ammonium treatment
[0102] Under the double-whammy condition of high-fat diet and vitamin D deficiency (HFD+VDD), mice develop typical symptoms of metabolic syndrome. In the background section we described the mechanism of metabolic syndrome. It is generally believed that a high-calorie (high-fat, high-sugar) diet is an important cause of metabolic syndrome. At the same time, a large number of clinical studies and animal experiments have shown that in addition to high calorie, a "second hit" is needed to convert common fatty liver into steatohepatitis (NASH). Our recent work showed that vitamin D deficiency (VDD) can effectively promote steatohepatitis induced by a high-fat diet [25]. The vitamin D (VD3, 1000IU / kg, ANI93 standard feed) contained in the normal feed can effectively inhibit the fatty liver caused by the high-fat diet. Under the c...
Embodiment 2
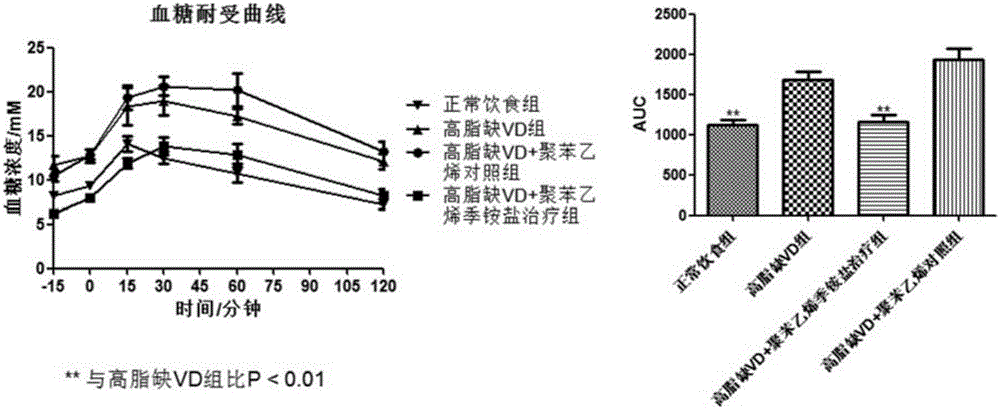
[0122] Embodiment 2, polystyrene quaternary ammonium treatment controls blood sugar tolerance
[0123] Fasting blood glucose and glucose intolerance are one of the main symptoms of metabolic syndrome. Continuously elevated fasting blood sugar is the main driving force for the formation of fatty liver. Blood sugar intolerance is one of the important indicators of diabetes. Describes failures and obstructions in the delivery of insulin to the system. Such as image 3 As shown, the degree of blood sugar intolerance can be measured by measuring the body's blood sugar tolerance curve (IPGTT / AUC). After the model mice were fasted for 6 hours, a certain dose of glucose was injected intraperitoneally, and the blood glucose tolerance curve was measured. On a high-fat vitamin D-deficient diet, mice developed typical blood sugar intolerance. High-fat diet plus vitamin D deficiency can effectively increase fasting blood sugar; at the same time, blood sugar tolerance experiments also ...
Embodiment 3
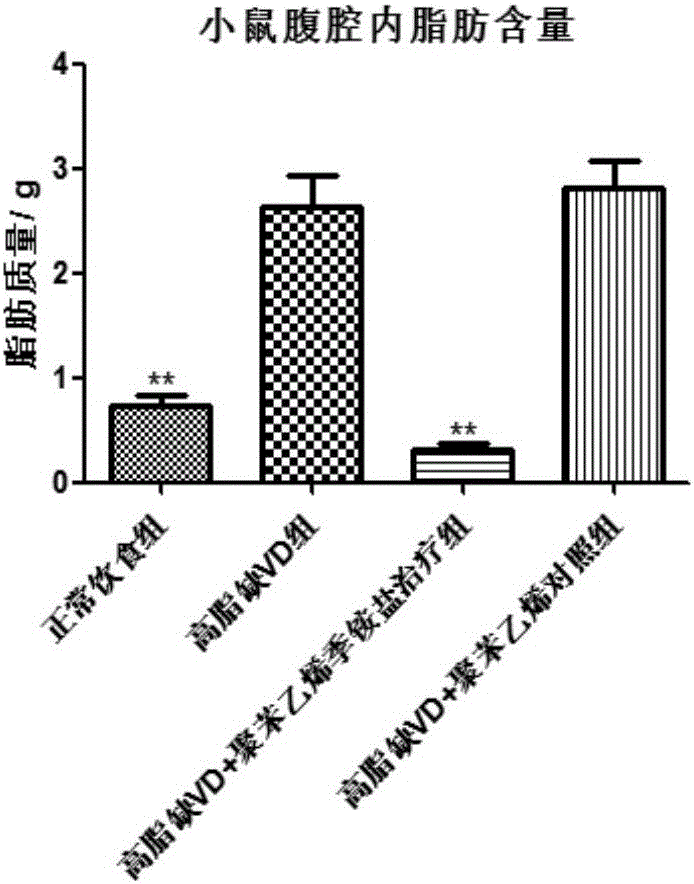
[0129] Embodiment 3, polystyrene quaternary ammonium treatment reduces intra-abdominal fat content
[0130]Another important indicator of metabolic syndrome is central obesity (central obese), and a large accumulation of abdominal fat. Central obesity is manifested in increased abdominal fat content, thicker waist circumference, and increased blood lipids. Different from subcutaneous fat, abdominal fat is the main tissue that produces systemic inflammation. There are a large number of innate immune cells in abdominal fat tissue, such as macrophages (macrophages) are the main tissue that produces TNF-alpha. Persistent systemic inflammation is the main cause of insulin resistance. Therefore, persistent low-grade inflammation, insulin resistance, makes blood sugar rise; glucose in blood cannot enter target organs, including skeletal muscle, subcutaneous fat tissue. As a result, a large amount of glucose enters the liver, and the glucose in the liver can be converted into fatty ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com