Amide bond containing heterocyclic compound, preparation method and application thereof
A technology for heterocyclic compounds and compounds, which is applied in the fields of amide bond-containing heterocyclic compounds and their preparation and application, and can solve the problems of poor curative effect of leukemia and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
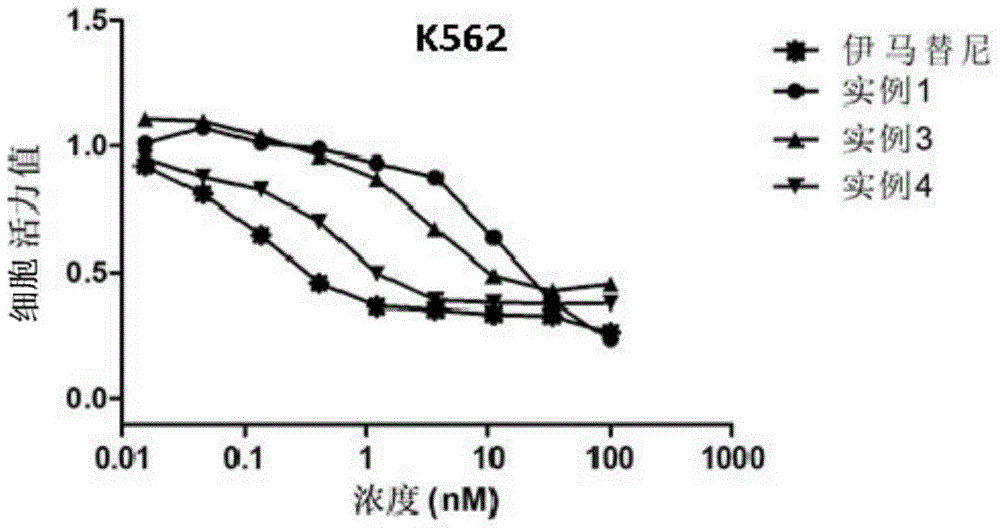
[0061] Example 1 Chemical Synthesis and Structure Identification of Heterocyclic Compounds Containing Amide Bonds with General Formula One
[0062] 1. General formula one structural formula is as follows:
[0063]
[0064] In the above general formula one, the substituent-R includes the following structures:
[0065]
[0066] 2. The chemical synthesis experimental procedure of the heterocyclic compound containing amide bond with general formula one (experimental procedure of the example compound contained in general formula one)
example 1
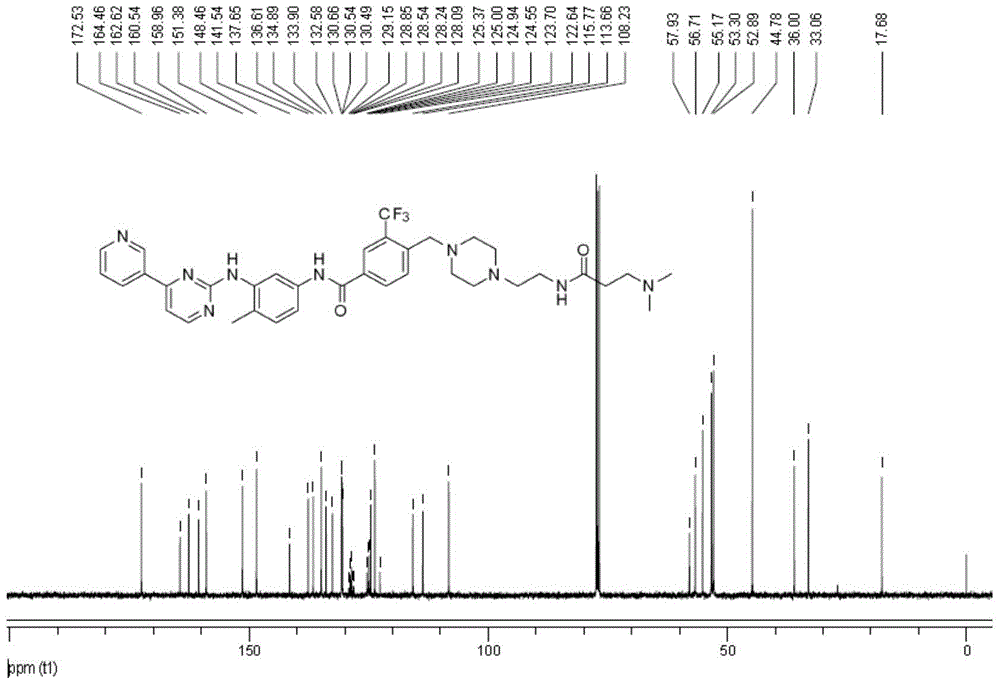
[0067] Example 1 Compound 7a: 4-((4-(2-aminoethyl)piperazin-1-yl)methyl)-N-(4-methyl-3-((4-(pyridine-3-yl) Synthesis and Structure Identification of Pyrimidin-2-yl)amino)phenyl)-3-(trifluoromethyl)benzamide
[0068]
[0069] 2.2.1.1: Compound 4: 4-bromomethyl-N-(4-methyl-3-{[4-(pyridin-3-yl)pyrimidin-2-yl]amine}phenyl)-3-trifluoro Synthesis of methyl benzamide
[0070]
[0071] Compound 2 (621mg, 2.16mmol) was dissolved in 12mL of anhydrous dichloromethane, stirred at 0°C for 5 minutes, 553uL oxalyl chloride and 20uL DMF were added to the reaction solution in turn, the reaction solution was stirred at 0°C for 3 hours, and the reaction solvent was removed with a rotary evaporator , the residue was dissolved in 10 mL of anhydrous THF, 598 mg of potassium carbonate was added, compound 1 (500 mg, 1.8 mmol) was dissolved in 20 mL of THF and added dropwise to the reaction system, and stirred at room temperature for 6 hours. After the reaction was completed, 100 mL of water w...
example 2
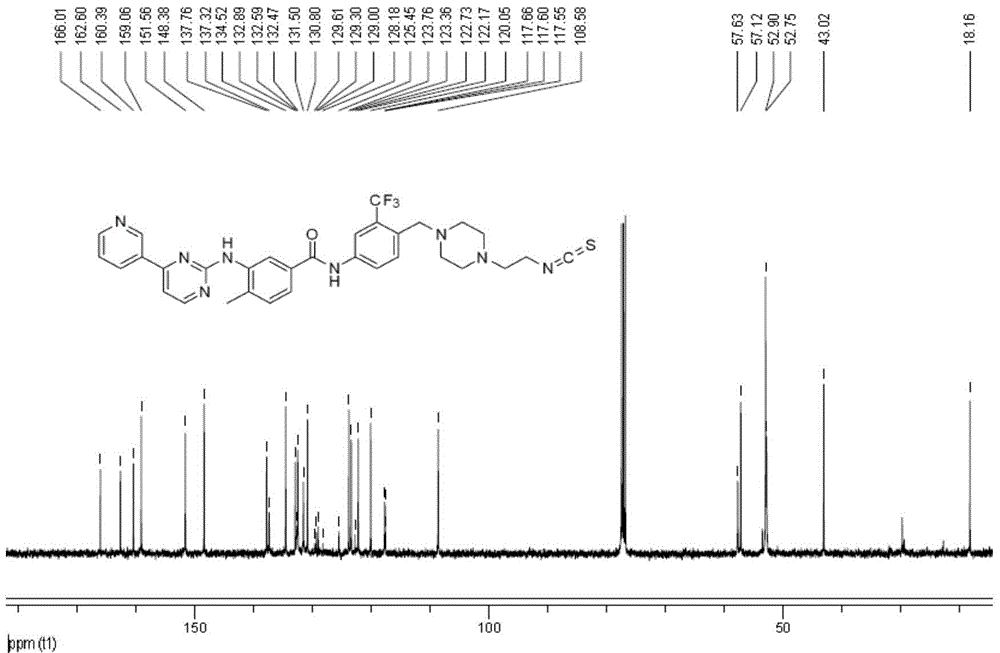
[0081] Example 2 compound 7b: 4-((4-(2-isothiocyanate ethyl) piperazin-1-yl) methyl)-N-(4-methyl-3-((4-(pyridine- Synthesis and Structure Identification of 3-yl)pyrimidin-2-yl)amino)phenyl)-3-(trifluoromethyl)benzamide
[0082]
[0083] Under nitrogen protection, 8ml THF was added to a 25mL three-necked flask, stirred at 0°C for 5 minutes, compound 7a (270mg, 0.457mmol), carbon disulfide (348mg, 4.57mmol) and DCC (N,N'-dicyclohexylcarbonyl Amine, 94.3mg, 0.457mmol), react at 0°C for 2 hours. After the reaction, the solvent was removed by spin, and the residue was purified by column chromatography to obtain 191.5 mg of white solid, yield: 66.2%.
[0084] HRMS-ESI(+): C 32 h 31 f 3 N 8 The calculated value of OSH is 633.2549, and the measured value is 633.2485;
[0085] 1 H NMR (400MHz, CDCl 3 ): δppm 9.23(d, J=1.6Hz, 1H), 8.67(dd, J=1.2, 4.8Hz, 1H), 8.59(s, 1H), 8.49(d, J=5.2Hz, 1H), 8.46( d,J=8.0Hz,1H),8.16(s,1H),8.12(s,1H),8.01(d,J=8.0Hz,1H),7.92(d,J=7.6Hz,1H),7.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com