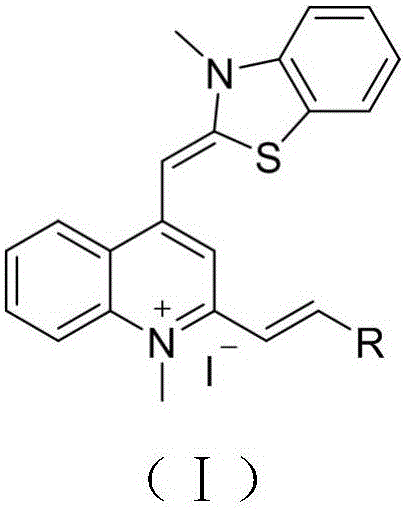
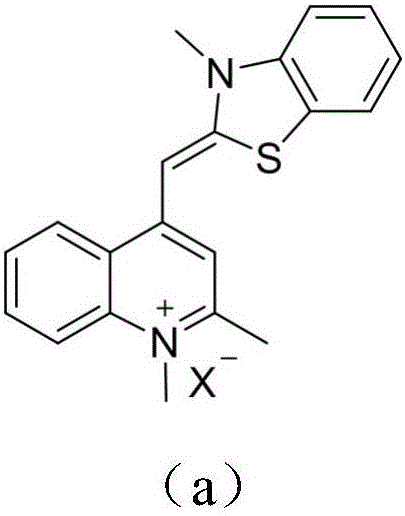
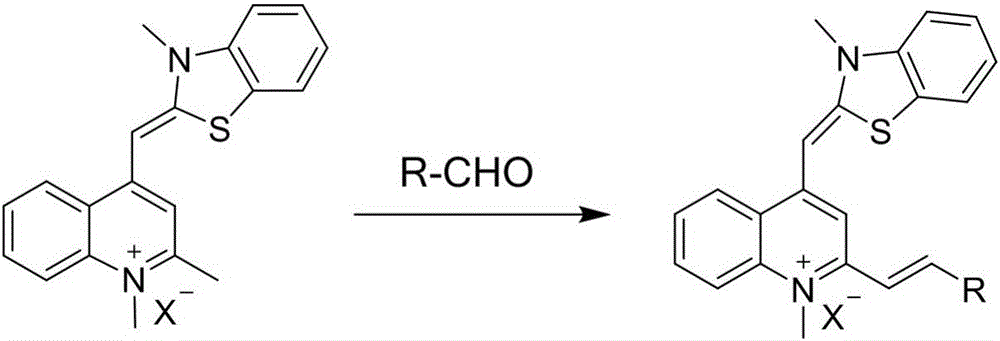
Thiazole orange styrene derivative, method for preparing same and application of thiazole orange styrene derivative to preparing medicines capable of resisting medicine-resistant bacteria
A technology for thiazole orange styrene and derivatives, which is applied in the field of new pharmaceutical compounds and achieves the effects of cheap raw materials, simple preparation method and significant inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Synthesis of Thiazole Orange Styrene Derivatives
[0030] Weigh out 0.15mmol In a 25ml round-bottomed flask, add 0.30mmol p-hydroxybenzaldehyde, 1.5ml n-butanol and 5 drops of 4-methylpiperidine, react at 130-135°C for 3 hours, cool to room temperature, and collect the crude product by suction filtration. Recrystallization of n-butanol to obtain a purple-black solid powder compound is a thiazole orange styrene derivative, and its chemical structural formula is as shown in formula (II):
[0031]
[0032] Yield 88%; 1 H NMR (400MHz, DMSO) δ8.59 (t, J = 17.8Hz, 1H), 8.04–7.82 (m, 3H), 7.79–7.60 (m, 3H), 7.49 (d, J = 22.2Hz, 3H) ,7.36(t,J=18.0Hz,3H),6.87(d,J=7.9Hz,2H),6.68(s,1H),3.98(d,J=22.1Hz,3H),3.83(s,3H) .13CNMR(100MHz,DMSO)δ160.39(s), 159.11(s), 152.57(s), 147.55(s), 141.65(s), 140.78(s), 139.26(s), 133.45(s), 131.04 (s), 128.37(s), 126.71(s), 125.49(s), 124.38(s), 124.09(s), 123.73(s), 123.32(s), 118.79(s), 117.93(s), 116.25 (s), 112.79(s), 107.92...
Embodiment 2
[0033] Example 2: Synthesis of Thiazole Orange Styrene Derivatives
[0034] The preparation method of this embodiment except using Except for replacing p-hydroxybenzaldehyde, all the other are the same as in Example 1. Finally, the purple-red solid powder compound is a thiazole orange styrene derivative, and its chemical structural formula is as shown in formula (Ⅲ):
[0035]
[0036] Yield 83%; 1 H NMR (400MHz, DMSO) δ8.72(d, J=8.4Hz, 1H), 8.24(d, J=8.68Hz, 1H), 8.08(d, 1H), 8.02(d, J=7.4Hz, 3H ),7.84(d,J=4.5Hz,1H),7.75(m,J=9.5Hz,3H),7.65(s,1H),7.56(s,3H),7.45(t,J=8.3Hz,1H ),6.95(s,1H),4.27(s,3H),4.02(s,3H). 13CNMR (100MHz, DMSO) δ159.57(s), 154.63(s), 148.40(s), 140.95(s), 139.64(s), 139.49(s), 133.58(s), 130.62(s), 129.43( s), 128.50(s), 126.83(s), 125.82(s), 124.67(s), 124.14(s), 123.91(s), 123.48(s), 123.17(s), 118.73(s), 113.10( s), 111.03(s), 87.33(s), 37.56(s), 34.09(s).ESI-MS: [M–I] + (C 27 h 22 ClN 2 S + ): theoretical value m / z 441.1, actual value: m / z...
Embodiment 3
[0037] Example 3: Synthesis of Thiazole Orange Styrene Derivatives
[0038] The preparation method of this embodiment except using Except for replacing p-Hydroxybenzaldehyde, all the other are the same as in Example 1. Finally, the reddish-brown solid powder compound is a thiazole orange styrene derivative, and its chemical structural formula is as shown in formula (IV):
[0039]
[0040] Yield 85%; 1 H NMR (400MHz, DMSO) δ8.69 (d, J = 8.4Hz, 1H), 8.01 (dd, J = 29.3, 10.1Hz, 4H), 7.94–7.89 (m, 1H), 7.70 (d, J = 7.6Hz, 1H), 7.63(dd, J=19.6, 11.2Hz, 3H), 7.54(d, J=14.5Hz, 1H), 7.44(s, 1H), 7.34(dd, J=19.2, 8.3Hz, 3H), 6.79(s,1H), 4.07(s,3H), 3.92(s,3H). 13 C NMR (100MHz, DMSO) δ159.83(s), 152.07(s), 148.00(s), 140.82(s), 139.79(s), 139.32(s), 133.66(s), 132.22(s), 131.24 (s), 131.16(s), 128.49(s), 126.90(s), 125.61(s), 124.66(s), 124.27(s), 123.85(s), 123.42(s), 121.96(s), 118.89 (s), 116.47(s), 116.25(s), 113.09(s), 108.23(s), 88.29(s), 38.53(s), 34.13(s).ESI-MS: [MI] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com