Calcareous purple soil phosphorus grading method
A grading method and calcareous technology, applied in thermal excitation analysis, material excitation analysis, etc., can solve problems such as the study of limiting phosphorus cycle, and achieve the effect of ensuring scientificity and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
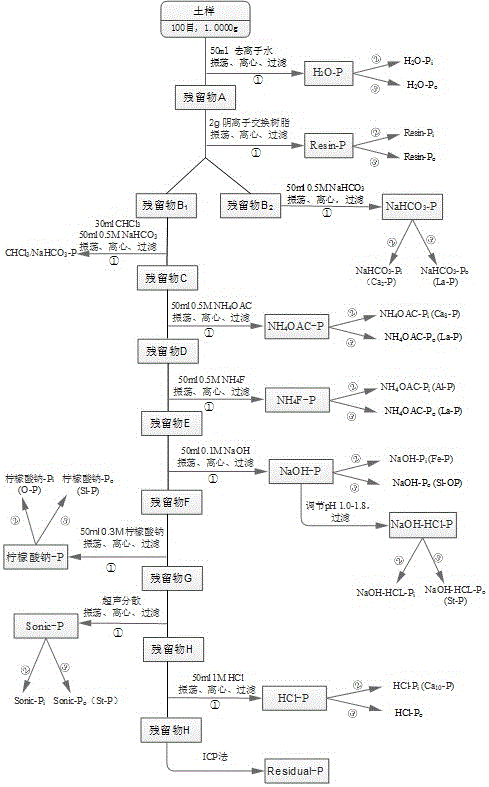
[0020] The method for grading phosphorus in calcareous purple soil of the present invention first collects a sample of calcareous purple soil and air-dries it through a 100-mesh sieve, then uses a chemical extractant to extract step by step, that is, uses different chemical reagents to extract organic phosphorus and inorganic phosphorus in the soil sample step by step. Phosphorus component, concrete steps are as follows:
[0021] 1) Use deionized water to extract water-soluble phosphorus H in soil samples 2 O-P, including water-soluble organophosphorus H 2 O-P o and water-soluble inorganic phosphorus H 2 O-P i ;
[0022] 2) In the soil sample extracted in step 1), use anion exchange resin to extract easily convertible phosphorus in the soil sample, that is, resin-soluble phosphorus Resin-P, including resin-soluble organic phosphorus Resin-P o And resin soluble inorganic phosphorus Resin-P i ;
[0023] 3) Add sodium bicarbonate NaHCO to the soil sample extracted in step ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com