Butanedione reductase application
A diacetyl and reductase technology, which is used in the introduction of foreign genetic material using a carrier, recombinant DNA technology, fermentation, etc., can solve the problems of various applications of enzymatic properties, such as the lack of further research, and achieve substrate specificity. Good, high enzymatic activity, broad prospects for industrial applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
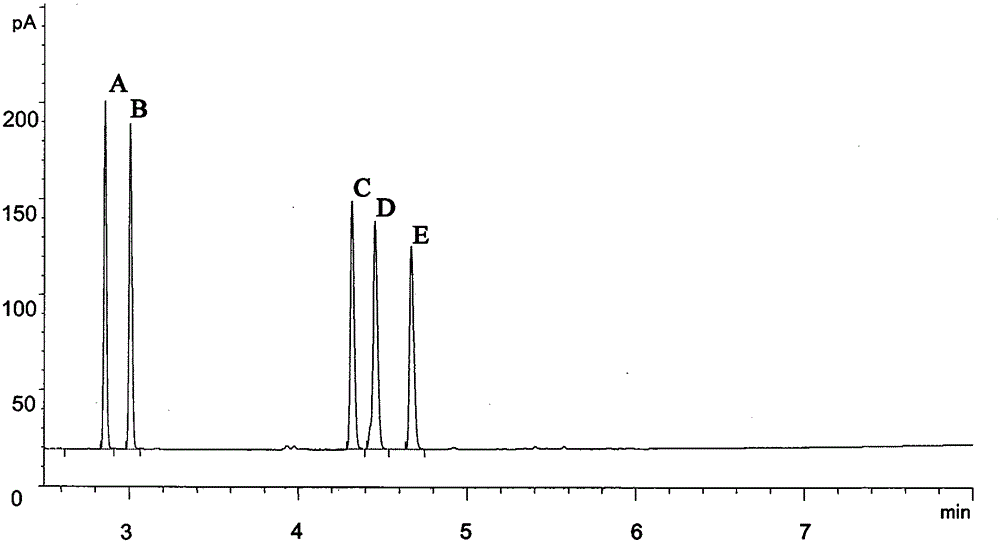
[0061] Example 1 Preparation of (S)-acetoin, (R)-acetoin, (S, S)-2,3-butanediol, meso-2,3-butanediol and ethyl lactate.
[0062] Using pETDuet-1 as the starting vector, the fragment of the gene encoding diacetyl reductase bdh obtained by PCR was double digested, and then connected to pETDuet-1 that had been treated by the same double digestion to construct pETDuet-bdh. On this basis, the same method was used to construct pETDuet-bdh-fdh. Introduce pETDuet-bdh-fdh into Escherichia coli E.coli BL21(DE3) to obtain recombinant Escherichia coli E.coli BL21(DE3)(pETDuet-bdh-fdh). Pick positive single clones and shake to OD at 37°C 600 = 0.6-0.8, adding 0.2mM IPTG, inducing at 25°C for 10h, collecting the cells by centrifugation, resuspending in binding buffer (containing 20mM phosphate, 300mM NaCl, 10mM imidazole, pH7.4), and ultrasonically disrupting the cells.
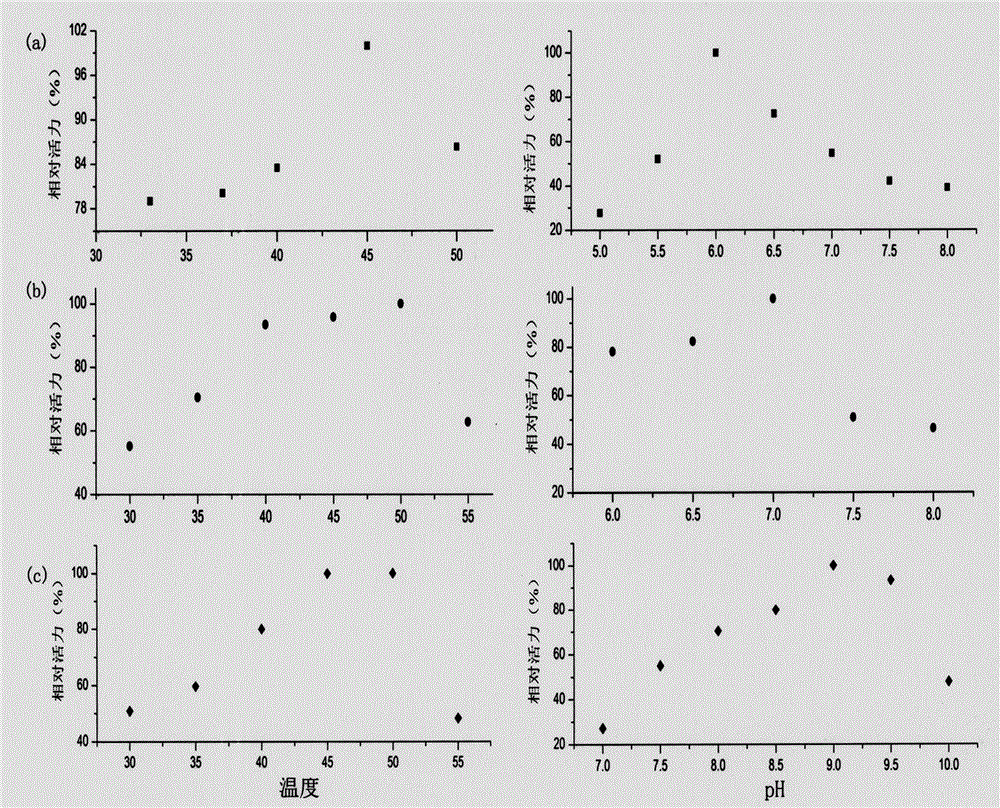
[0063] Carry out protein purification by His-tag label, specific method is: obtain supernatant through Ni-NTA gel col...
Embodiment 2
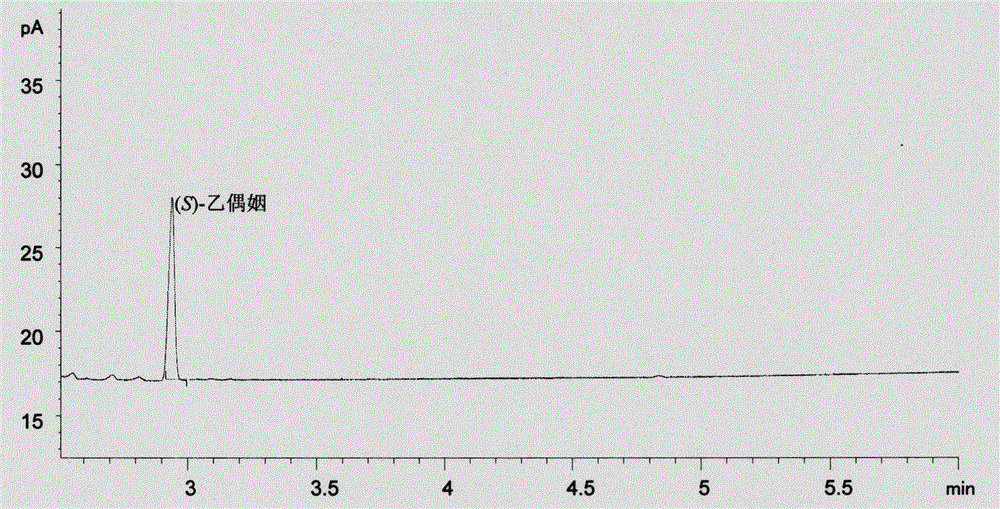
[0068] Example 2 Application of recombinant Escherichia coli containing diacetyl reductase BDH encoding gene bdh to catalyze diacetyl synthesis (S)-acetoin
[0069]1) Using pETDuet-1 as the starting vector, construct the recombinant E. coli expression vector pETDuet-bdh-fdh containing the gene bdh encoding diacetyl reductase and the gene fdh encoding formate dehydrogenase, and introduce it into E. coli BL21(DE3) , to obtain recombinant Escherichia coli E.coli BL21(DE3) / pETDuet-bdh-fdh;
[0070] 2) After a single colony of E.coli BL21(DE3) / pETDuet-bdh-fdh was transferred to LB liquid medium for activation culture, protein expression was induced at 25°C and 0.2mM IPTG to obtain Cells with enzyme BDH activity and formate dehydrogenase FDH activity as biocatalysts;
[0071] 3) Using the biocatalyst obtained in step 2), using diacetyl as a substrate, sodium formate as a hydrogen donor, and reacting at pH 6.0-9.0 at 33°C.
[0072] Gas chromatography was used to determine the conce...
Embodiment 3
[0073] Example 3 Application of recombinant Escherichia coli containing diacetyl reductase BDH encoding gene bdh to catalyze (S)-acetoin synthesis of (S, S)-2,3-butanediol
[0074] 1) Using pETDuet-1 as the starting vector, construct the recombinant E. coli expression vector pETDuet-bdh-fdh containing the gene bdh encoding diacetyl reductase and the gene fdh encoding formate dehydrogenase, and introduce it into E. coli BL21(DE3) , to obtain recombinant Escherichia coli E.coli BL21(DE3) / pETDuet-bdh-fdh;
[0075] 2) After a single colony of E.coli BL21(DE3) / pETDuet-bdh-fdh was transferred to LB liquid medium for activation culture, protein expression was induced at 25°C and 0.2mM IPTG to obtain Cells with enzyme BDH activity and formate dehydrogenase FDH activity as biocatalysts;
[0076] 3) Using the biocatalyst obtained in step 2), using (S)-acetoin as a substrate, sodium formate as a hydrogen donor, and reacting at pH 6.0-9.0 at 33°C.
[0077] The concentration and optical ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com