Method for detecting enantiomer in vildagliptin midbody
A technology of enantiomers and intermediates, which is applied in the field of high-performance liquid phase detection of enantiomers in -1--2-pyrrolidinecarbonitrile, can solve the problem of no detection method and peak shape symmetry Not good, can not be improved and other problems, to achieve good effect, strong practicability, easy to adjust the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] S3. Preparation of mixed solution:
[0045] Weigh (2S)-1-(2-chloroacetyl)-2-pyrrolidinecarbonitrile into the volumetric flask, accurately measure (2R)-1-(2-chloroacetyl)-2- Pyrrolidinecarbonitrile reference substance solution was added to the volumetric flask and diluted to the mark with mobile phase, mixed, shaken, and used as a mixed solution for later use. According to the mass-volume ratio (g / L), the ratio of (2S)-1-(2-chloroacetyl)-2-pyrrolidinecarbonitrile: mobile phase is 0.1~5:1, and the optimized ratio is 1:1; According to the mass-volume ratio (g / L), the ratio of (2R)-1-(2-chloroacetyl)-2-pyrrolidinecarbonitrile: mobile phase is 0.001~0.1:1, and the optimized ratio is 0.01:1;
[0046] S4, draw the reference substance solution, need testing solution and mixed solution of equal amount respectively, inject in high performance chromatograph and measure, the measuring condition of described high performance liquid chromatography comprises:
[0047] Inspection met...
Embodiment 1
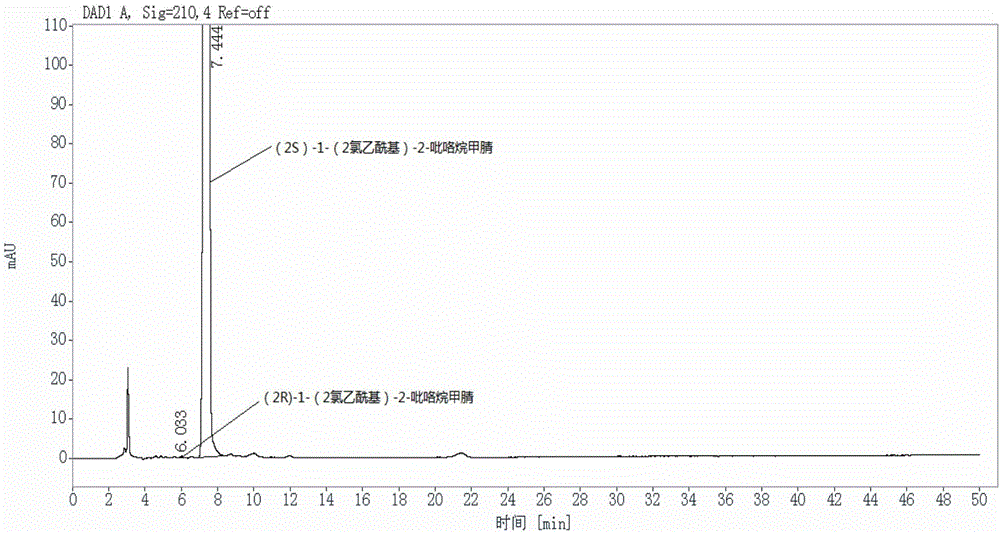
[0057] The (2S)-1-(2-chloroacetyl)-2-pyrrolidinecarbonitrile sample with batch number 160104 was selected for its (2R)-1-(2-chloroacetyl)-2-pyrrolidinecarbonitrile Content detection, including the following steps:
[0058] S1, prepare (2S)-1-(2-chloroacetyl)-2-pyrrolidinecarbonitrile test solution:
[0059] Weigh 25mg (2S)-1-(2-chloroacetyl)-2-pyrrolidinecarbonitrile sample into 25mL mobile phase, mix well, and prepare a solution containing 1.0mg of vildagliptin per 1mL, as the test product solution, ready to use.
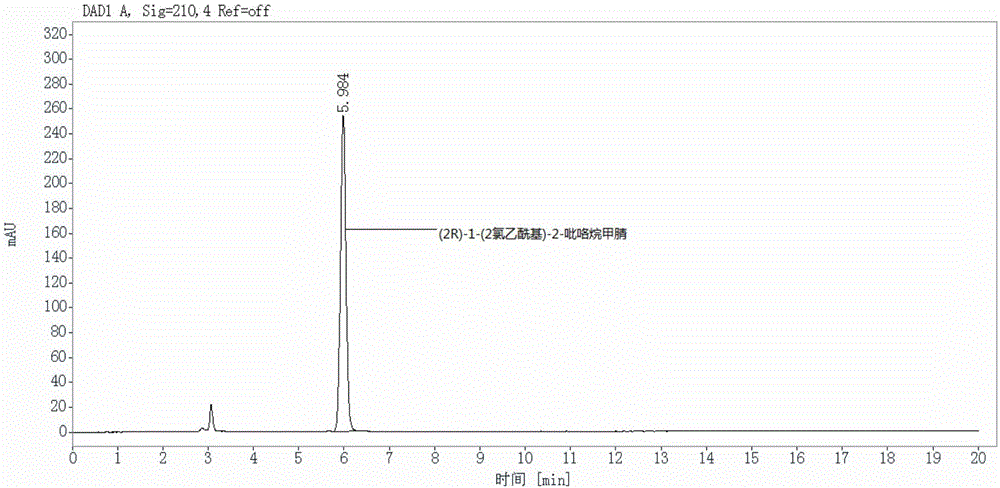
[0060] S2, prepare (2R)-1-(2-chloroacetyl)-2-pyrrolidinecarbonitrile reference substance solution:
[0061] Weigh an appropriate amount of (2R)-1-(2-chloroacetyl)-2-pyrrolidinecarbonitrile reference substance, accurately weigh, dissolve in the mobile phase and quantitatively dilute to make a solution containing about 100 μg per 1 mL, as (2R )-1-(2-chloroacetyl)-2-pyrrolidinecarbonitrile reference solution.
[0062] S3. Preparation of mixed solution:
[0063] W...
Embodiment 2
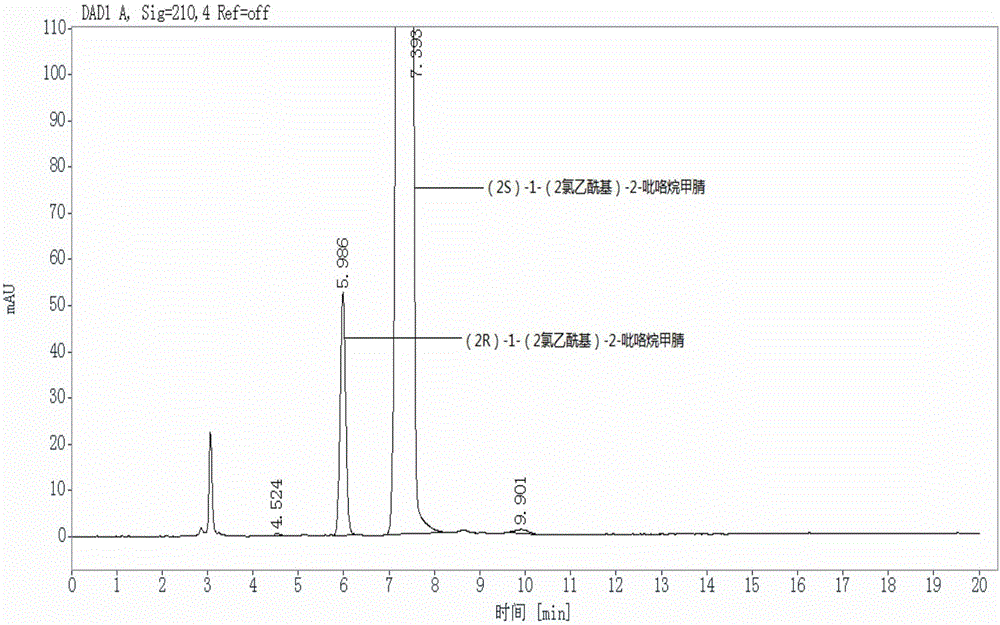
[0073] Select (2S)-1-(2-chloroacetyl)-2-pyrrolidinecarbonitrile samples with the same batch number as in Example 1, for (2R)-1-(2-chloroacetyl)-2-pyrrole The content of alkanecarbonitrile is detected. The difference between this example and Example 1 is only that the parameters of the flow rate in the chromatographic conditions are different, and other detection conditions are consistent with Example 1. In this embodiment, the determination conditions of high performance liquid chromatography include:
[0074] Chromatographic column: CHIRALPAK IC (model: length 250mm, inner diameter 4.6mm, cellulose surface covalently bonded silica filler, filler particle size 5μm);
[0075] Detector: UV detector;
[0076] Detection wavelength: 210nm;
[0077] Column temperature: 30℃;
[0078] Flow rate: 0.8mL / min;
[0079] Mobile phase: calculated by volume ratio, n-butanol:ethanol:diethylamine=60:40:0.05.
[0080] After testing, the content of (2R)-1-(2-chloroacetyl)-2-pyrrolidinecarbon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com