Application of naucleamide G in preparation of anti-inflammatory drug
An anti-inflammatory drug and inflammatory technology, applied in the field of medicine, can solve the problem of unclear active substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
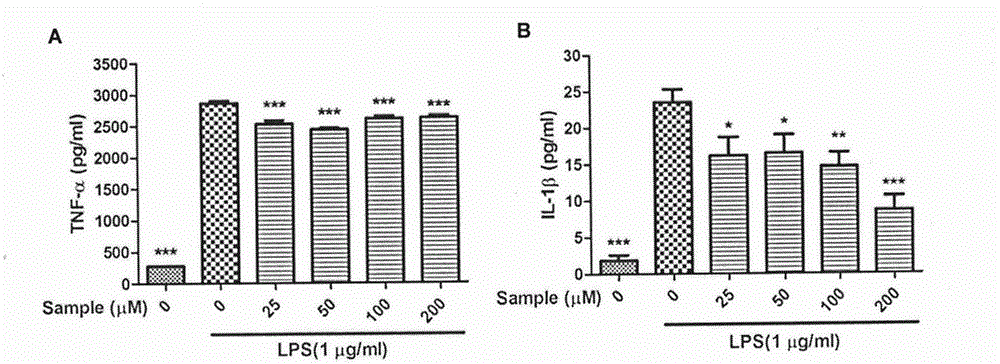
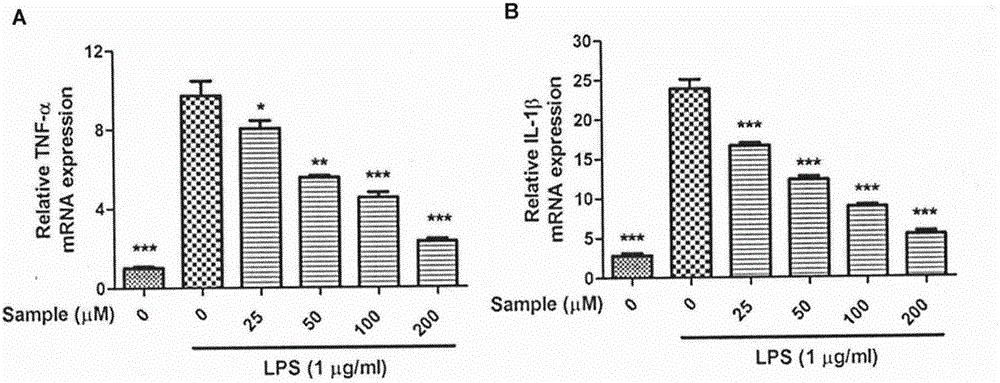
[0019] Example: The compound of the present invention, Naucleamide G, has a good inhibitory effect on inflammation in LPS-stimulated RAW264.7 cells.
[0020] (1) Cell culture
[0021] Mouse RAW 264.7 macrophages were purchased from Shanghai Cell Bank, Chinese Academy of Sciences. at 37°C, 5% CO 2 Condition, cultured with DMEM incomplete medium containing 10% HyClone fetal bovine serum, 100 U / mL penicillin and 100 mg / mL streptomycin, digested with 0.5% trypsin for passage. All operations are aseptic.
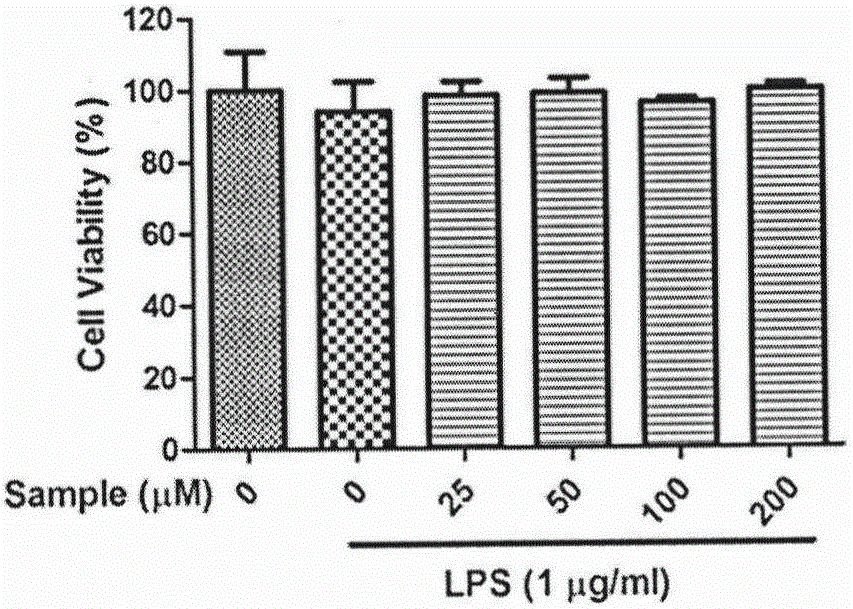
[0022] (2) Cell Viability Experiment
[0023] RAW 264.7 cells were seeded in 96-well plates (4×10 6 1 / mL), six replicate wells in each group, and each well contained 200 μL of incomplete high-glucose DMEM medium. After the cells adhered, the cells were washed with PBS once, the control group was added with incomplete high-glucose medium, the induction group was added with incomplete high-glucose medium containing 1 μg / mL LPS, and the administration group was added with 25, 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com