Ratio fluorescence probe capable of quickly and highly selectively analyzing hydrazine
A compound and linear technology, which is used in the field of ratio fluorescent probes for fast and high-selective analysis of hydrazine by naphthalimide compounds, can solve the problems of single fluorescence enhancement, quenching, and difficulty in eliminating background interference, and achieves synthesis Simple and stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
[0037] (Scheme 1) Dissolve 269mg (1.0mmol) of 4-hydroxy-1,8-naphthalimide and 165mg (1.0mmol) of 4-bromobutyric acid in 15mL of dichloromethane, add 98mg of DMAP (4-dimethylamino pyridine), 330mg DCC (dicyclohexylcarbodiimide) as the catalyst of the reaction, then stirred at room temperature for 6h, then rotary evaporated to obtain the crude product, and finally used the dichloromethane system to carry out column chromatography to obtain 359mg of the yellow pure product, the yield is 86%.
[0038] (Scheme 2) Dissolve 269mg (1.0mmol) of 4-hydroxy-1,8-naphthalimide and 182mg (1.1mmol) of 4-bromobutyric acid in 15mL of dichloromethane, add 97.6mg of DMAP (4-dimethyl aminopyridine), 329.6mg DCC (dicyclohexylcarbodiimide) as the catalyst of the reaction, then stirred at room temperature for 6h, and then rotary evaporated to obtain the crude product, and finally used the dichloromethane system to carry out column chromatography to obtain 367mg of the yellow pure product,...
Embodiment 2
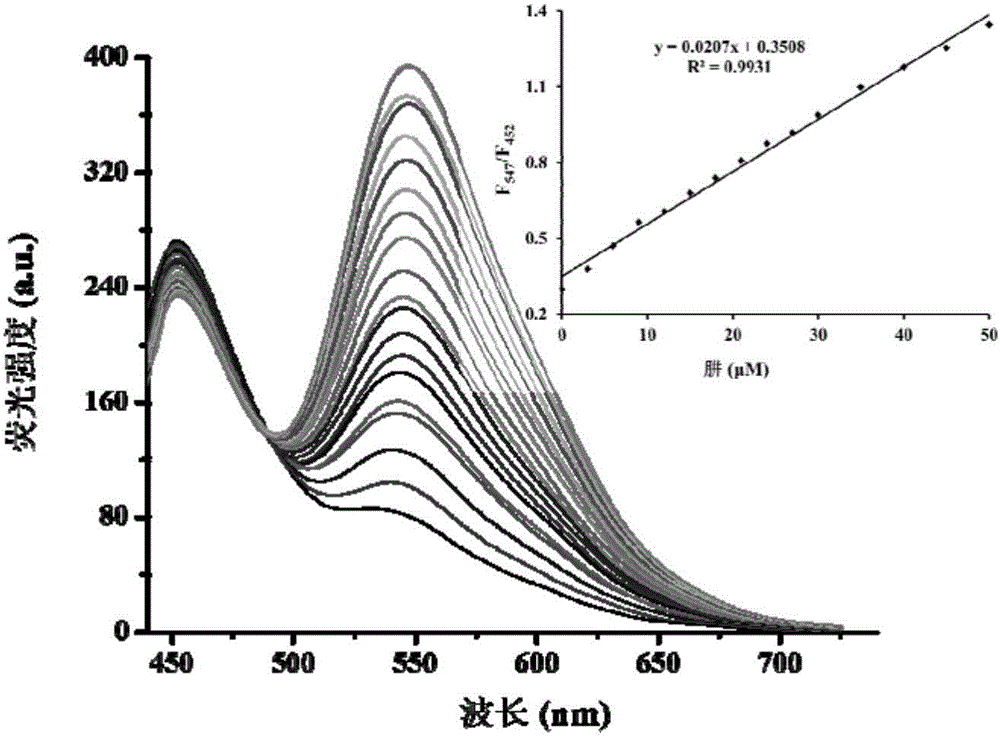
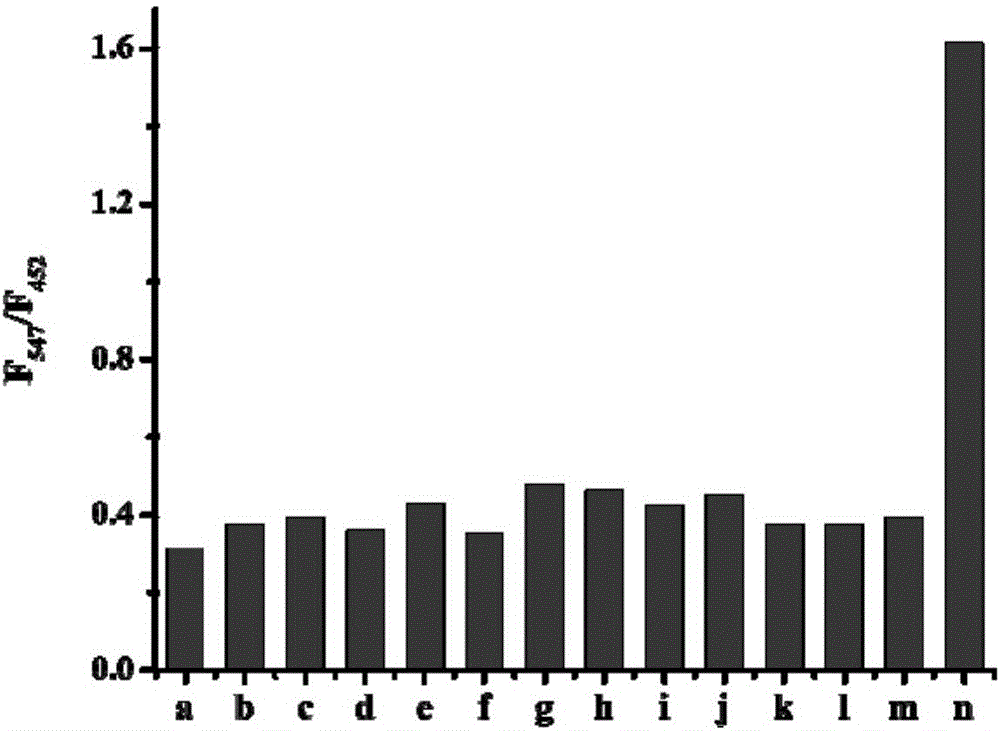
[0044] The effect of different concentrations of hydrazine (0-100μM) on the fluorescence spectrum of the probe (5μM). The above determination is carried out in a mixed system of absolute ethanol and water (v / v, 3:7), 5mM PBS, pH 7.4, the probe used is the probe prepared in Example 1, and all spectra The measurements were all made at 25°C 20 minutes after the addition of hydrazine. See results figure 1 .
[0045] Under the condition of not adding hydrazine, the probe solution has a main fluorescence emission peak at about 452nm. Under the condition of adding hydrazine (100 μM), the maximum fluorescence emission peak is at 547 nm. By increasing the hydrazine concentration (0-100 μM), we further tested the change of the fluorescence spectrum of the probe (5 μM). Such as figure 1 As shown, under the condition of adding hydrazine, we observed that the emission peak at 452nm decreased gradually and a new red-shifted emission peak at 547nm appeared synchronously. At the same ti...
Embodiment 3
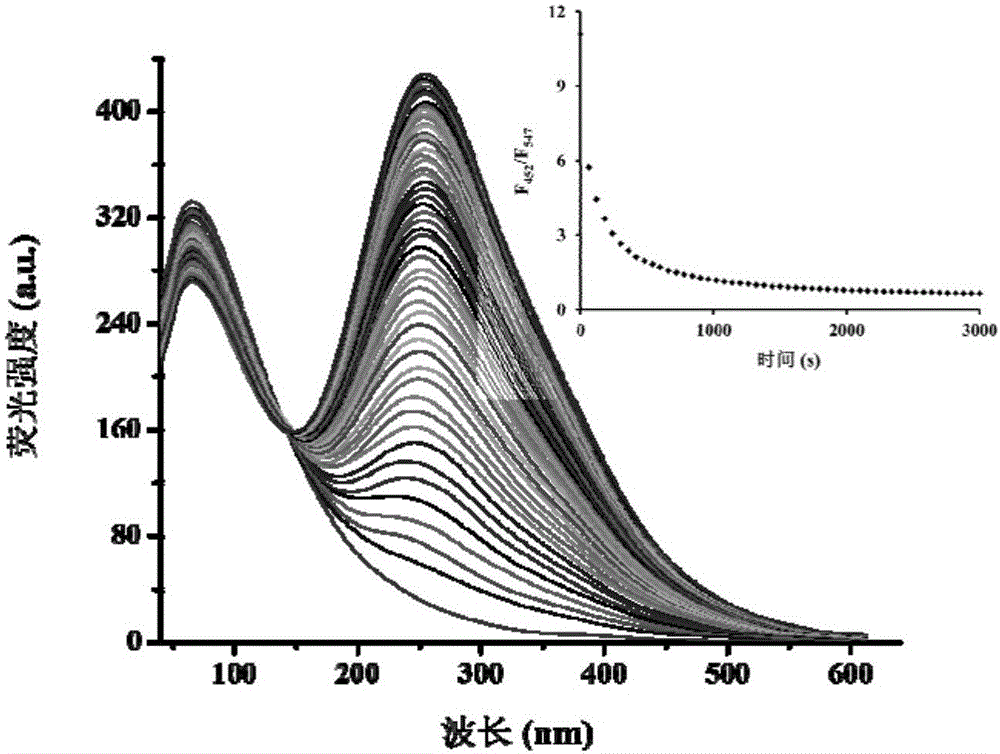
[0047] Test results of probe (5 μM) response time to hydrazine (100 μM). The above determination is carried out in a mixed system of absolute ethanol and water (v / v, 3:7), 5mM PBS, pH 7.4, the probe used is the probe prepared in Example 1, and all spectra The tests are all measured at 25°C. See results figure 2 .
[0048] From figure 2 It can be seen that the fluorescence ratio gradually decreases with the progress of the reaction, and the response time is about 20 minutes. The results showed that the response rate of the probe to hydrazine was faster than that of the reported fluorescent probes. Therefore, our synthesized probe can recognize hydrazine more quickly.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com