Highly selective ultra-sensitive colorimetric fluorescent probes for detecting copper ions
A hydrogen atom and compound technology, applied in the field of rhodamine compounds as copper ion fluorescent probes, can solve problems such as difficulty in copper ion detection, and achieve the effects of being conducive to popularization and application, simple synthesis and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
[0035] (Scheme 1) Dissolve 300mg (0.685mmol) rhodamine compounds in 15mL of dichloromethane, then add 253mg (2.055mmol) 2-pyridinecarboxylic acid to reflux for 1.5h, then utilize a high-pressure pump to carry out suction filtration to obtain the filtrate, and the filtrate Pass directly through the chromatographic column to obtain pure product. 163 mg of pink pure product was obtained with a yield of 44%.
[0036] (Scheme 2) Dissolve 300mg (0.685mmol) rhodamine compounds in 15mL of dichloromethane, then add 295mg (2.40mmol) 2-pyridinecarboxylic acid to reflux for 1.5h, then utilize a high-pressure pump to carry out suction filtration to obtain the filtrate, and the filtrate Pass directly through the chromatographic column to obtain pure product. 194mg of pink pure product was obtained with a yield of 52%.
[0037] (Scheme 3) 300mg (0.685mmol) rhodamine compounds were dissolved in 15mL of dichloromethane, then 337mg (2.74mmol) 2-pyridinecarboxylic acid was added an...
Embodiment 2
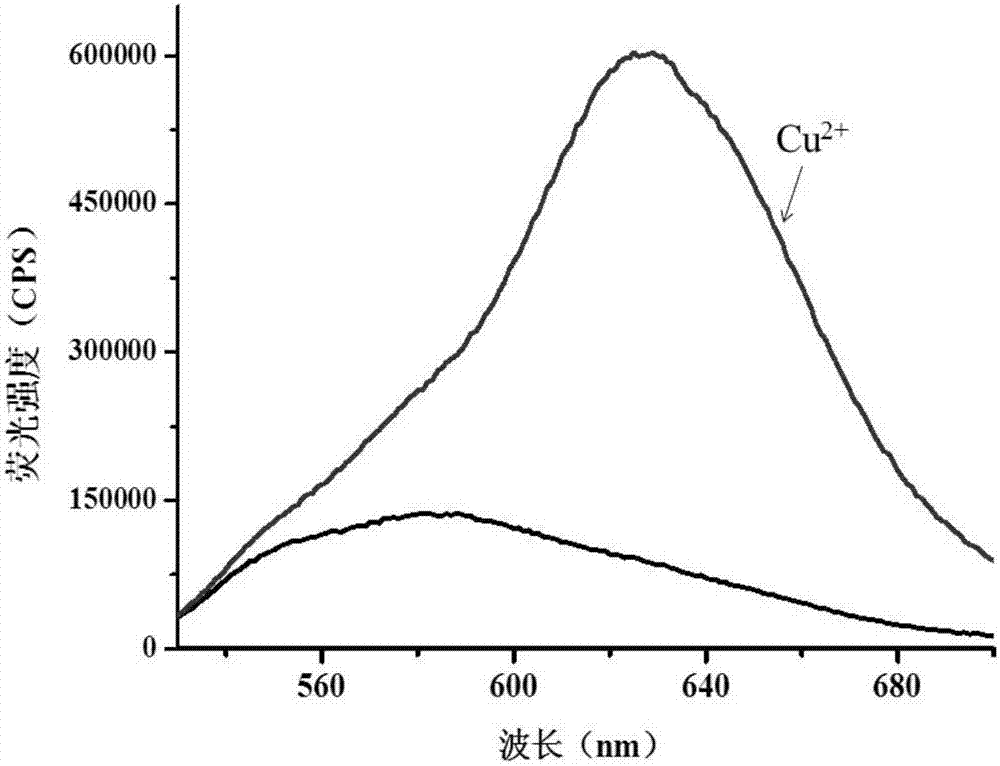
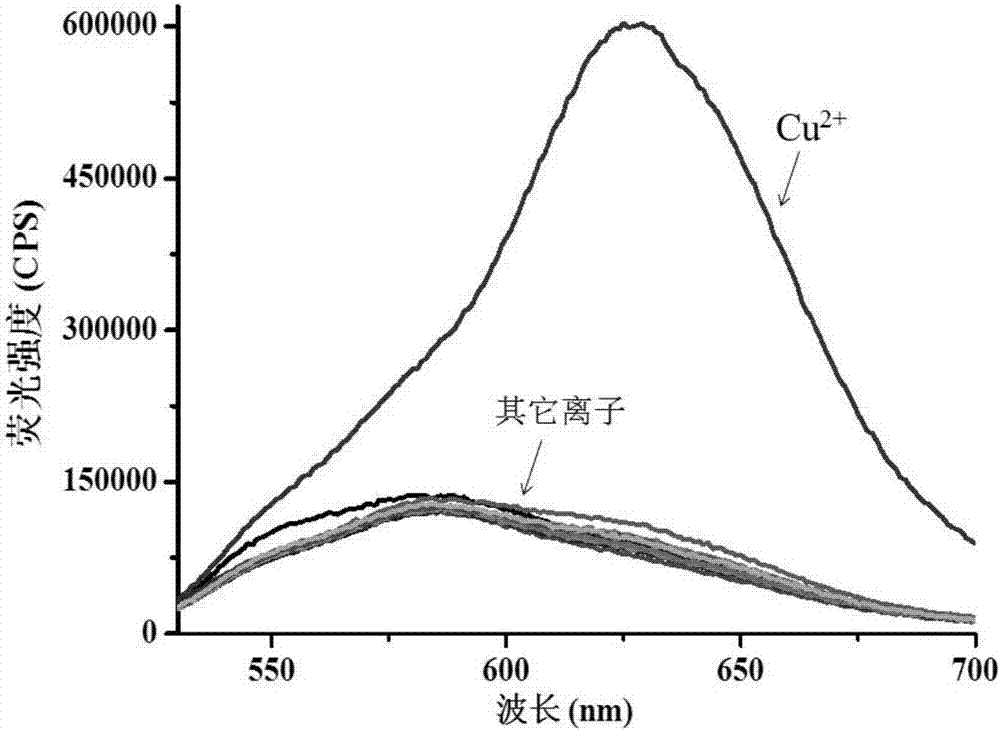
[0041] First prepare 1mM probe stock solution and 10mM copper ion stock solution. Add 5mL of distilled water to the washed colorimetric tube first, then use a pipette gun to draw 50μL of the probe stock solution into the tube, add 0.5mL of phosphate buffer solution (pH7.0), dilute to 10mL and mix well. It was then divided into two, and 5 μM copper ions were added to one of the tubes. After 15 minutes of response, the fluorescence intensity values were measured with a fluorescence spectrometer. Among them, the excitation wavelength is 515nm, the emission wavelength is 630nm, and the test temperature is 25°C. The results are as follows: figure 1 shown.
[0042] figure 1 Yes probe (5 μM) with Cu added 2+ (5μM) before and after the fluorescence spectrum, we can see that the fluorescence change is very obvious through the illustration, indicating that the probe has a good response to copper ions.
Embodiment 3
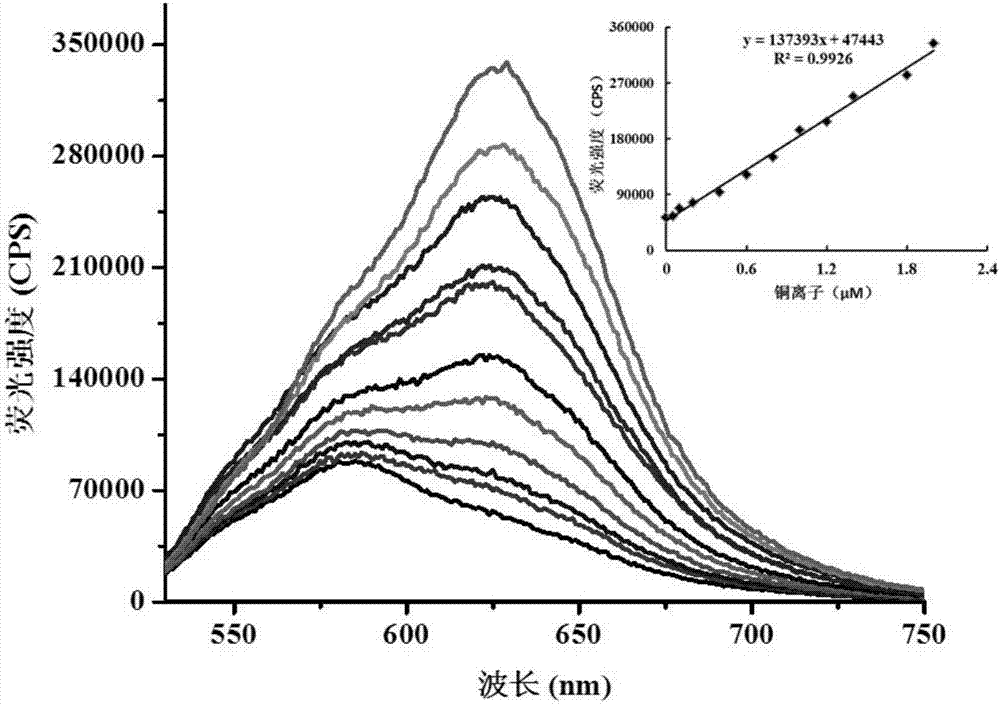
[0044] On the basis of the stock solution, prepare 150mL of 5μM probe solution in a beaker, transfer it to 12 clean colorimetric tubes, and calibrate accurately. Add the copper ion stock solution into the colorimetric tube so that its final concentrations are: 0, 0.1, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, 1.6, 1.8, 2.0 μM and shake it evenly. The test system is pure water, containing 5mM phosphate buffer solution (pH 7.0). After leaving the reaction for 15 minutes, measure its emission spectrum with a fluorescence spectrometer, record and save it. Among them, the excitation wavelength is 515nm, the emission wavelength is 630nm, and the test temperature is 25°C. The results are as follows: figure 2 shown.
[0045] figure 2 are different concentrations of Cu 2+ (0-2μM) on the probe (5μM) fluorescence spectrum;
[0046] It can be seen that with the Cu in the probe solution 2+ As the concentration increased, the fluorescence intensity gradually increased. The appropriate con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com