High-selectivity mercury ion near-infrared fluorescence probe taking phenyl thionocarbonate as recognition receptor
A technology based on ester groups and hydrogen atoms, which is applied in the field of mercury ion near-infrared fluorescent probes, can solve the problems of harsh reaction conditions, complex synthesis steps, and poor photostability, and achieve simple synthesis, convenient detection and operation, and good stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
[0037] (Scheme 1) Dissolve 500 mg (1.49 mmol) of fluorescent dye, 516 mg (2.98 mmol) of phenyl thiochloroformate (384 mg, 2.98 mmol) and N,N-diisopropylethylamine (DIPEA) in 15 mL of dichloromethane , then stirred and reacted at 25°C for 9h, and then rotary evaporated to obtain the crude product, and finally used dichloromethane:methanol=60:1 system for column chromatography to obtain 647mg of pure product with a yield of 92%.
[0038](Scheme 2) Dissolve 500 mg (1.49 mmol) of fluorescent dye, 540 mg (3.13 mmol) of phenyl thiochloroformate (384 mg, 2.98 mmol) and N,N-diisopropylethylamine (DIPEA) in 15 mL of dichloromethane , then stirred and reacted at 25°C for 9h, and then rotary evaporated to obtain the crude product, and finally used dichloromethane:methanol=60:1 system for column chromatography to obtain 645mg of pure product with a yield of 92%.
[0039] (Scheme 3) Dissolve 500 mg (1.49 mmol) of fluorescent dye, 257 mg (1.49 mmol) of phenyl thiochloroformate (...
Embodiment 2
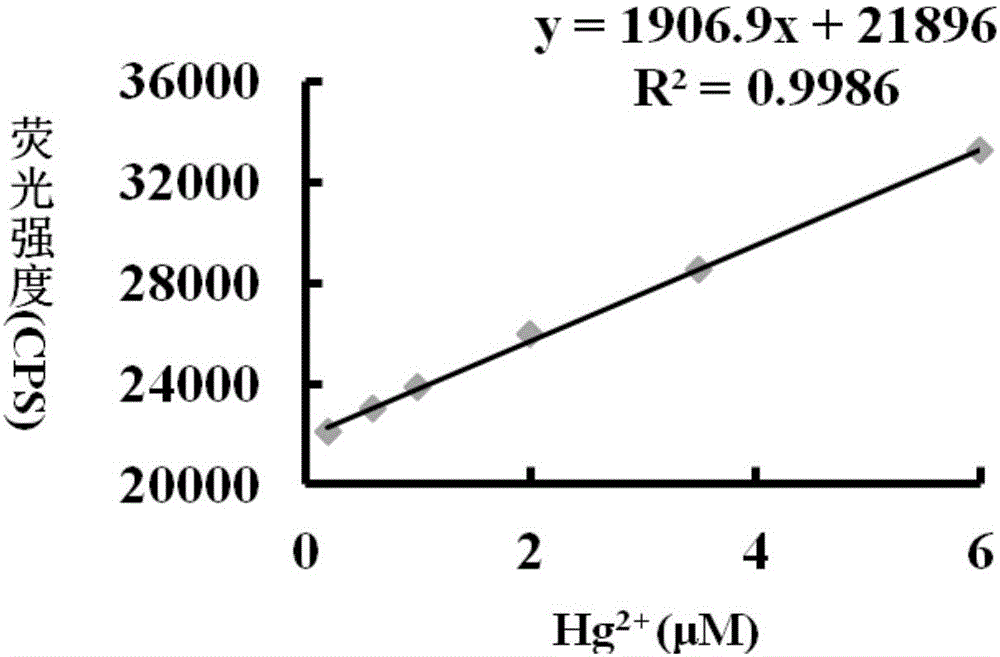
[0043] Different concentrations of Hg 2+ (0-10μM) on the fluorescence spectrum of the probe (5μM). The above determinations were carried out in an aqueous solution of 5 mM HEPES, pH 7.0, the probe used was the probe prepared in Example 1, and all spectral tests were measured at 25° C. for 20 min. See results figure 1 .
[0044] From figure 1 It can be seen that with the Hg in the probe solution 2+ As the concentration increases, the fluorescence intensity gradually increases, and at 0-10μM Hg 2+ Concentration range, Hg 2+ The concentration and fluorescence intensity showed a linear relationship. Therefore, the probe of the present invention can more accurately determine the content of mercury ions in the sample to be tested.
Embodiment 3
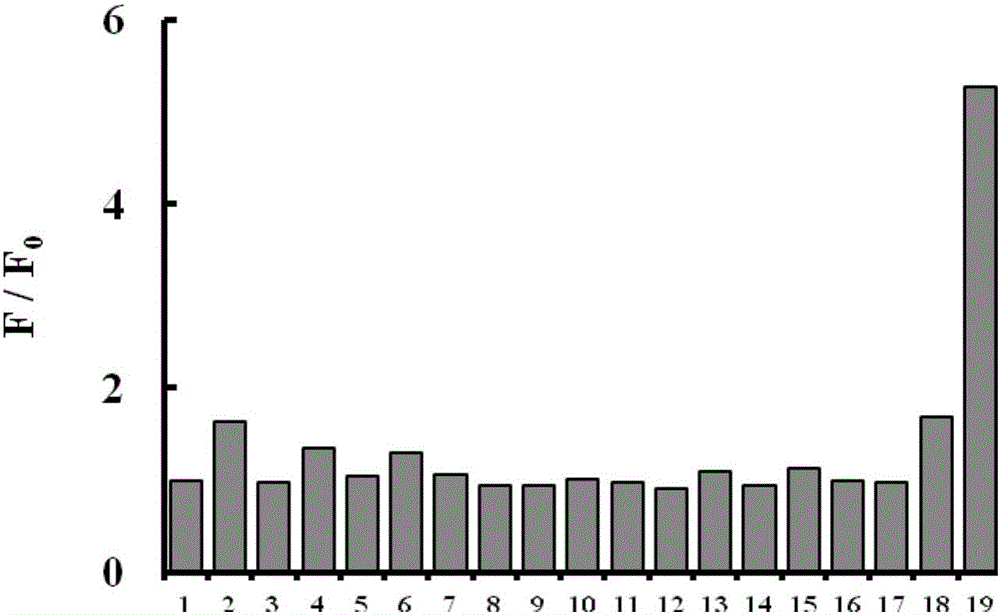
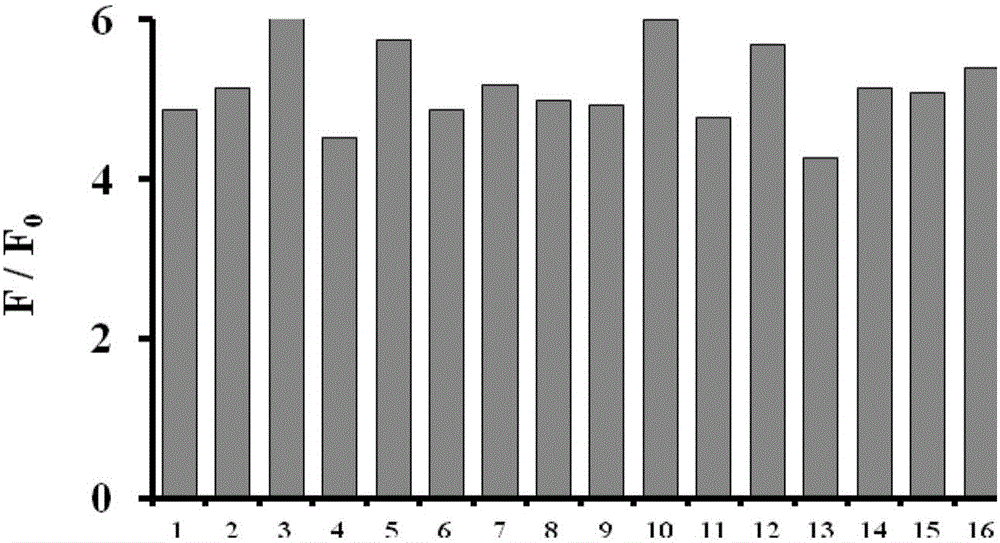
[0046] Fig. 2(a) Effect of different analytes on the fluorescence spectrum of the probe (5 μM). (b) Quantitative analysis of Hg with different analyte pair probes (5 μM) by fluorescence spectrometry 2+ (6μM). Analytes include: Potassium ion K + , Sodium ion Na + , calcium ion Ca 2+ , Magnesium ion Mg 2+ , ferrous ion Fe 2+ , iron ion Fe 3+ , aluminum ion Al 3+ , silver ion Ag + , copper ion Cu 2+ , Cadmium ion Cd 2+ , lead ion Pb 2 + , Zinc ion Zn 2+ , fluoride ion F - , nitrate ion NO 3 - , nitrite ion NO 2 - , Carbonate CO 3 2- , bicarbonate HCO 3 - , Chloride ion Cl - , Sulfate ion SO 4 2- , sulfite ion SO 3 2- , bisulfite ion HSO 3 - , Bromide Br - , and their concentration is 50 μM. All test conditions are carried out in 5mM HEPES, pH 7.0 aqueous solution, the probe used is the probe prepared in Example 1, and all spectral tests are measured after acting at 25°C for 20 minutes. The results are shown in Figure 2(a) and (b). Specifically, pip...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com