Oleanolic acid-3-one derivative having antitumor effects, preparation method, and application thereof
An anti-tumor effect, oleanolic acid technology, applied in the field of medicine, can solve the problems of weak activity, limited application, low bioavailability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
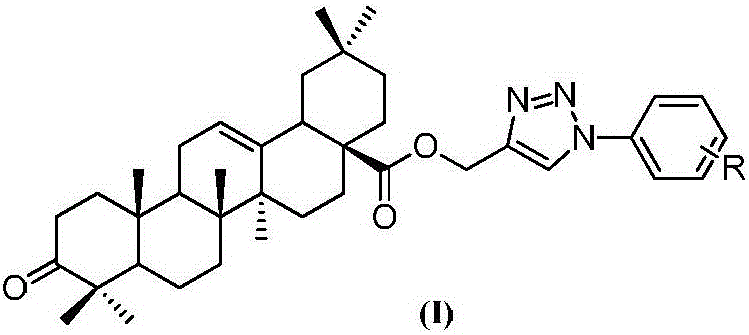
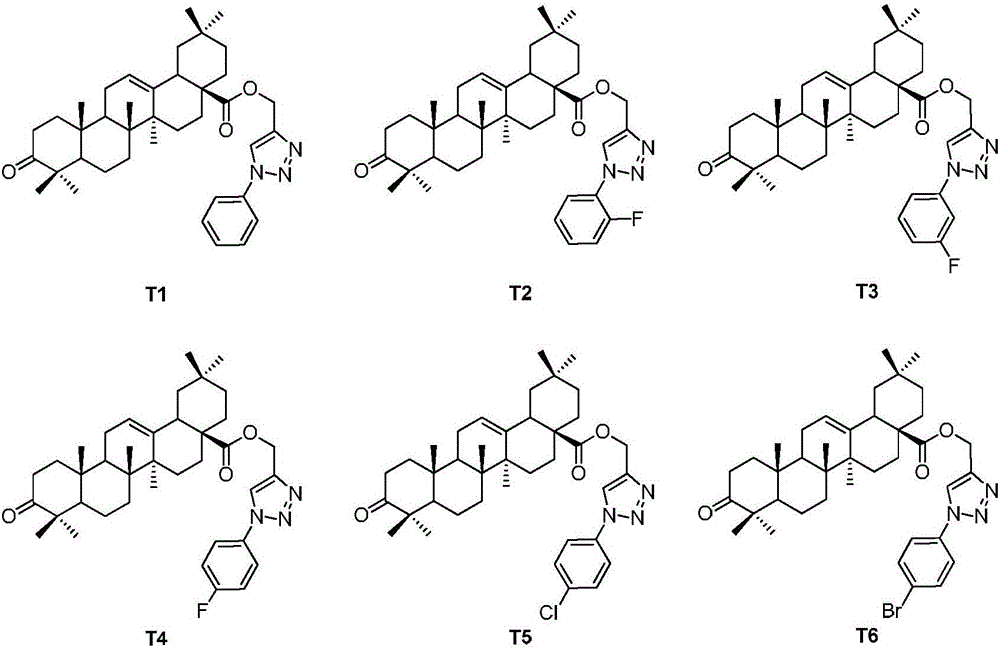
[0027] Example 1: Oleanolic acid-3-keto-(1'-phenyl-1H-1',2',3'-triazol-4'-yl)methyl ester (T1)
[0028] Oleanolic acid 1 (2.0g, 4.4mmol), K 2 CO 3 Powder (1.8g, 13.1mmol) and TBAB (290.0mg, 0.9mmol) were dissolved in a mixed solution of 1mL water and 200mL dichloromethane, propargyl bromide (1.0g, 8.8mmol) was added, and the reaction was stirred at room temperature for 5h, Water washing, saturated NaCl washing, anhydrous NaSO 4 Drying, filtration, concentration, and column chromatography (petroleum ether: ethyl acetate = 9:1) yielded 1.93 g of white solid 2 with a yield of 89.1%.
[0029] Compound 2 (3.0 g, 6.0 mmol) was dissolved in 50 mL of acetone, and Jones reagent (2.3 mL, 12.0 mmol) was slowly added dropwise at 0°C. Stir the reaction for 30 minutes, filter with suction, distill off acetone, add 80 mL of ethyl acetate to dissolve, and wash with water (2×10 mL). The organic layer was concentrated and purified by column chromatography (petroleum ether: ethyl acetate = 5...
Embodiment 2
[0032] Example 2: Oleanolic acid-3-keto-[1'-(2"-fluorophenyl)-1H-1',2',3'-triazol-4'-yl]methyl ester (T2 )
[0033] The preparation method of the compound in Example 2 was the same as in Example 1, except that o-fluorophenyl azide was used instead of phenyl azide to obtain T2 as a yellow solid with a yield of 88.2%.
[0034] Mp 177.5-179.6°C; 1 H NMR (600MHz, CDCl 3 )δ8.45(d, J=8.9Hz, 2H), 8.19(s, 1H), 8.00(d, J=8.9Hz, 2H), 5.32(d, J=20.0Hz, 3H), 2.90(dd, J=13.7,3.3Hz,1H),2.58-2.51(m,1H),1.14(s,3H),1.09(s,3H),1.02(s,3H),0.94(s,3H),0.94(s ,3H),0.92(s,3H),0.55(s,3H); 13 C NMR (150MHz, CDCl 3)δ217.49,177.86,147.32,144.69,143.58,141.04,126.35,125.54,122.47,122.34,120.47,57.11,55.27,47.41,46.83,46.76,45.74,41.81,41.47,39.27,39.10,36.70,34.10,33.77,33.02 ,32.37,32.16,30.66,29.70,27.62,26.38,25.66,23.57,23.44,22.97,22.69,21.44,19.49,16.65,14.88,14.12; ESI-MS(m / z):652.8[M+Na] + .
Embodiment 3
[0035] Example 3: Oleanolic acid-3-keto-[1'-(3"-fluorophenyl)-1H-1',2',3'-triazol-4'-yl]methyl ester (T3 )
[0036] The preparation method of the compound in Example 3 was the same as in Example 1, except that m-fluorophenyl azide was used instead of phenyl azide to obtain white solid T3 with a yield of 84.2%.
[0037] Mp 167.2-170.1°C; 1 H NMR (600MHz, CDCl 3 )δ8.05(s,1H),7.71(d,J=8.8Hz,2H),7.54(d,J=8.8Hz,2H),5.34-5.28(m,3H),2.90(dd,J=13.6 ,3.8Hz,1H),2.57-2.51(m,1H),1.14(s,3H),1.09(s,3H),1.04(s,3H),0.94(s,3H),0.94(s,3H) ,0.92(s,3H),0.52(s,3H); 13 C NMR (150MHz, CDCl 3 )δ217.59,177.80,144.03,143.63,135.43,134.69,129.94,122.47,122.30,121.60,57.26,55.24,47.39,46.78,45.78,41.80,41.47,39.26,39.10,36.70,34.11,33.80,33.04,32.37,32.15 ,30.67,29.70,27.62,26.46,25.65,23.58,23.44,22.96,22.69,21.43,19.51,16.66,14.88,14.12; ESI-MS(m / z):652.6[M+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com