Method for screening anti-tumor metastasis compounds
A technology for anti-tumor metastasis and compounds, which is applied in the field of screening anti-tumor metastasis compounds, and can solve the problems that cannot be used for screening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
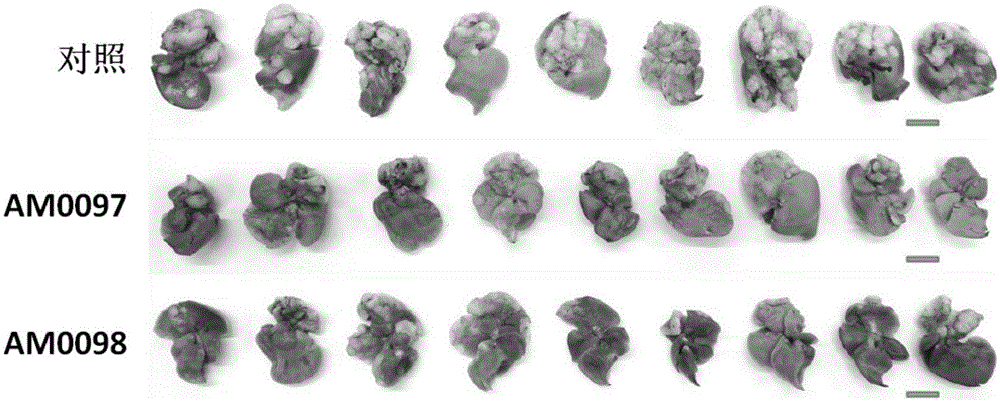
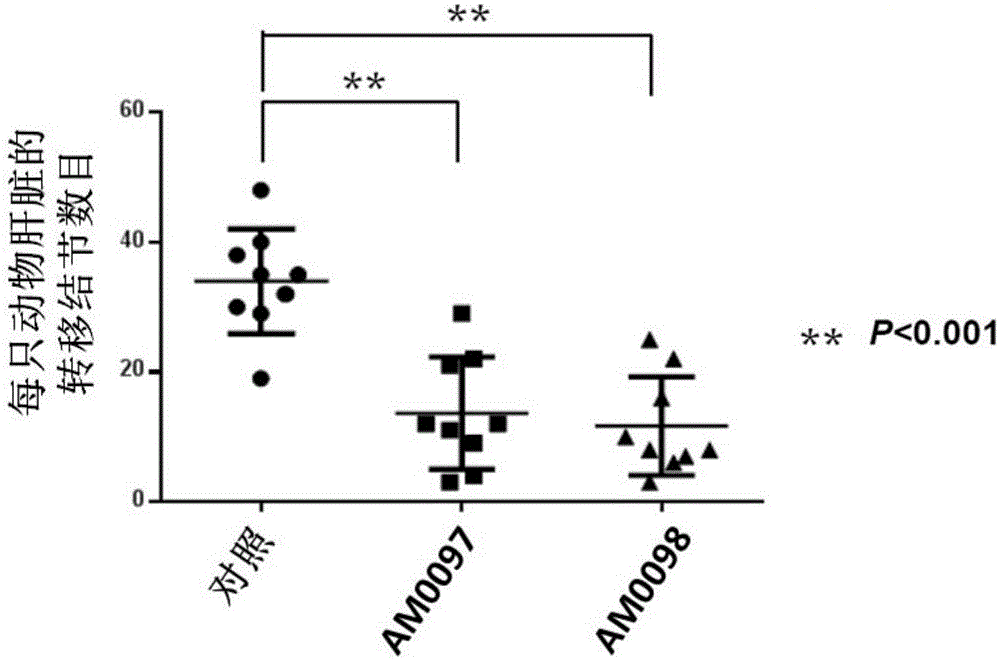
[0029] The present invention uses the highly metastatic cell line S18 to establish a spleen-liver metastasis nude mouse model (spleen-livermetastasis nude mouse model), and then gives the cells two anti-metastasis treatments (AM0097 and AM0098) designed by the inventors, about one One month later, the livers of all animals were taken out at the same time to calculate the number of metastatic nodules, such as figure 1 , 2 As shown, there was a statistically significant difference compared with the control, indicating that both treatments had anti-metastatic properties.
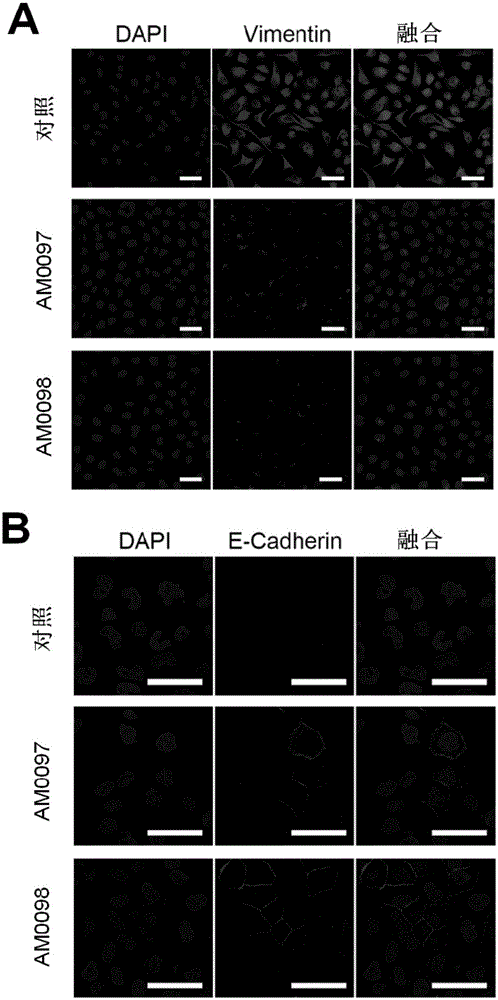
[0030] Cell immunofluorescence staining experiments showed that the expression of EMT markers in the S18 cells treated with two anti-metastatic treatments changed significantly in two-dimensional culture: the expression of the mesenchymal marker Vimentin was significantly reduced, and the expression of the epithelial marker E-cadherin was significantly reduced. Significantly increased (scale bar, 50μm), such a...
Embodiment 2
[0032] An embodiment of the method for screening compounds for anti-tumor metastasis of the present invention, the method comprises the following steps:
[0033] (1) Construct tumor cells capable of quantitatively displaying the EMT process, and measure the expression levels of EMT markers of the tumor cells, and the EMT markers include markers representing epithelial characteristics and markers representing mesenchymal; wherein the representative Epithelial markers (epithelial markers) are one or more combinations of E-Cadherin, Desmoplakin, Cytokeratins, Occludin and Claudin; the mesenchymal markers (mesenchymal markers) are Vimentin, N-cadherin, One or more combinations of Fibronectin, Snail1, Snail2, Twist1, Twist2, ZEB1, ZEB2, LAMC2, and Bmi-1, and the assay method is immunofluorescence assay or direct assay of fluorescein.
[0034] (2) co-incubating the compound to be tested with the tumor cells in the step (1) for 1-48 hours;
[0035] (3) After incubation, determine th...
Embodiment 3
[0038] An embodiment of the method for screening compounds for anti-tumor metastasis of the present invention, the method comprises the following steps:
[0039](1) Construct tumor cells capable of quantitatively displaying the EMT process, and measure the expression levels of EMT markers of the tumor cells, and the EMT markers include markers representing epithelial characteristics and markers representing mesenchymal; wherein the representative The epithelial markers (epithelial markers) are one or more combinations of E-Cadherin, Desmoplakin, Cytokeratins, Occludin and Claudin; the mesenchymal markers (mesenchymal markers) are Vimentin, N-cadherin , Fibronectin, Snail1, Snail2, Twist1, Twist2, ZEB1, ZEB2, LAMC2 and Bmi-1, the assay method is immunofluorescence assay.
[0040] (2) Plant the tumor cells in step (1) in a 24-well plate, a 96-well plate or a 384-well plate, add various compounds before or after cell attachment, and incubate together for 1-48 hours.
[0041] (3)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com