Use of 3-isoxazolidinones compounds as selective herbicides
A technology of isoxazolidinone and herbicide, which is applied in the direction of herbicide, algicide, biocide, animal repellent, etc., and can solve problems such as evolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
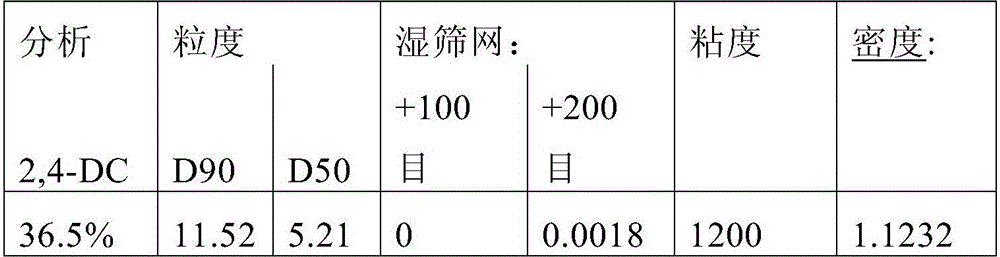
[0142] Example 1: Suspension concentrate (SC) formulation of 2-(2,4-dichlorophenyl)methyl-4,4-dimethyl-3-isoxazolidinone (2,4-DC)
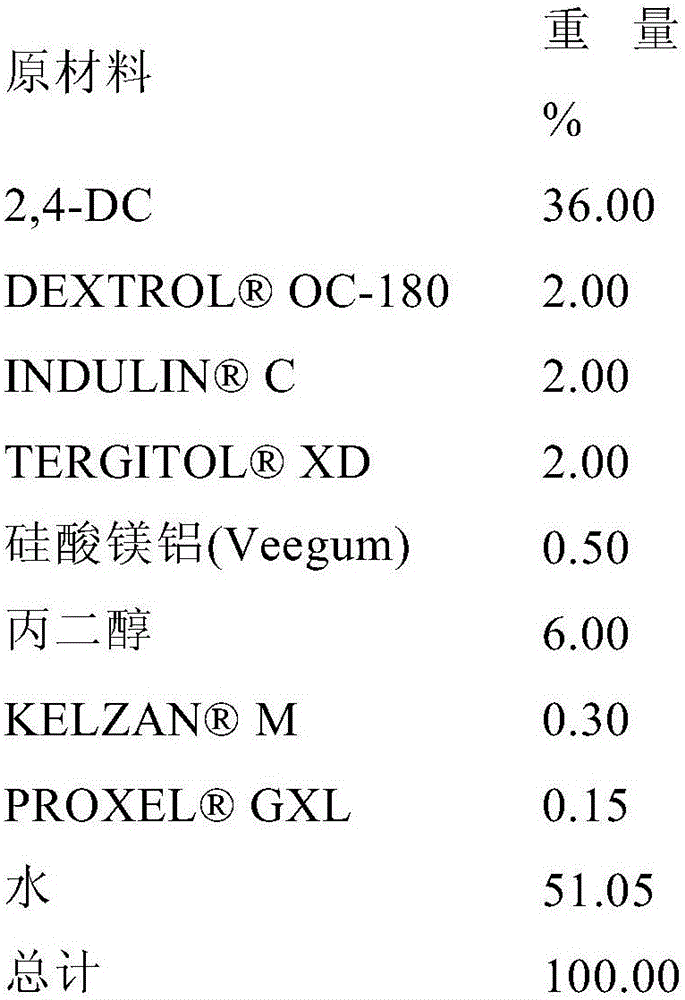
[0143] Formulation 1A: An SC formulation of 2,4-DC was prepared by mixing 37.89% 2,4-DC, 1.50% TERGITOL TM XD (Dow Chemical Company), 1.50% DEXTROL TM OC-180 (Ashland Specialty Company), 1.00% C, 0.10% AFE 100, 6.0% propylene glycol, 0.15% M Xanthan gum (CPKelco A Huber Company), 0.15% GXL, and 42.00% water (% by weight).
[0144] In at least one embodiment, the following microencapsulation process is used to prepare the formulations of the present invention: prior to use, in a 55°C oven using a slurry and discharge vessel, an attritor, melting XD.
[0145] Preparation of KELZAN / diol slurry:
[0146] Weigh the propylene glycol into a separate container. While stirring vigorously, slowly add KELZAN M. Mix until smooth.
[0147] Prepare pre-ground slurry:
[0148] Add water to mixing container and add to tank AFE-100, XD, OC-...
Embodiment 2
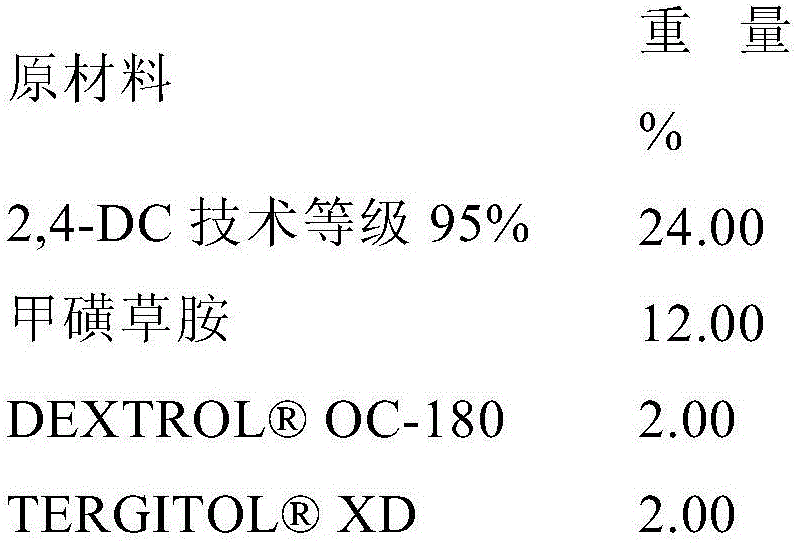
[0165] Example 2: Evaluation of pre-emergence weed control by 2-(2,4-dichlorophenyl)methyl-4,4-dimethyl-3-isoxazolidinone in mixtures with metazachlor
[0166] The herbicidal efficiency of the compositions herein was tested in the following manner:
[0167] Dilute with water containing 2,4 DC (Example 1B) and metazachlor ( S, 43.1% active ingredient, BASF) test composition and 2-(2,4-dichlorophenyl)methyl-4,4-dimethyl-3-isoxazolidinone and metazachlor mixture to provide the appropriate test rate concentration.
[0168] The crops tested were wheat and oilseed rape (Brassica napus), and the weeds tested were ryegrass multiflora (IR) (ryegrass, Polygonum multiflorum (Lolium perenne.multiflorum)), bluegrass (AB) (Poa annua ( Poaannua), Chenopodium (Chenopodium album)), Chickweed (CC) (Stellaria media), and Corn Poppy (CP) ((Papaver rhhoeas) poppies).
[0169] For pre-emergence testing, for each herbicide solution's individual application score, 4 disposable fiber panels (6" x...
Embodiment 3
[0177] Example 3: Evaluation of pre-emergence weed control by 2-(2,4-dichlorophenyl)methyl-4,4-dimethyl-3-isoxazolidinone in mixtures with napropamide
[0178] The herbicidal efficiency of the compositions herein was tested in the following manner:
[0179] Dilute with water containing 2,4 DC (Example 1C) and napropamide ( 50-DF, 50% active ingredient, test composition of United Phosphorus Inc. and 2-(2,4-dichlorophenyl)methyl-4,4-dimethyl-3- A mixture of isoxazolidinones and napropamide to provide the appropriate concentration for the test rate.
[0180] The crops tested were oilseed rape (Brassica napus) and wheat, and the weeds tested were ryegrass multiflora (IR) (ryegrass, Polygonum multiflorum (Lolium perenne.multiflorum)), bluegrass (AB) (Poa annua ( Poaannua)), rushes (BG) ((Alopecurus myosuroides)), wild buckwheat (WB) (Polygonum convolvulus), chickweed (CC) (Stellaria media), small seedlings (LC) (( Phalaris minor)) and wild oats (WO) (Avena fatua).
[0181] For...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com