Enzymatic hydrolysis of disaccharides and oligosaccharides using alpha-glucosidase enzymes
A technology of glucosidase and glucan, applied in oligosaccharide, hydrolase, glycosylase, etc., can solve problems such as low-rate substrate concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0292] Preparation of Syrup by Polymerizing Sucrose
[0293] This example discloses a general approach to producing a mixture of soluble sugars by polymerizing sucrose using the gtf enzyme in a glucan synthesis reaction. Specifically, a filtrate of the dextran synthesis reaction was prepared, which was then concentrated into a syrup.
[0294] Sucrose (3000 g) was added to a clean 5-gallon polyethylene bucket. Water (18.1L) and Fermasure TM (10 mL) was added to the barrel, and by adding 5 vol% NaOH and 5 vol% H 2 SO 4 Adjust the pH to 7.0. The final volume was about 20 L and the initial concentration of sucrose was 152.5 g / L as determined by HPLC. Dextran polymerization was initiated by adding 0.3% by volume of crude gtfase (SEQ ID NO: 3) extract prepared as described in the General Methods section. The extract contained approximately 2.9 mg / mL protein. Agitation was provided to the reaction solution using an overhead mechanical motor equipped with a glass shaft and PT...
Embodiment 2
[0301] Effects of enzymes on the hydrolysis of sugars in the filtrate of dextran synthesis reactions
[0302] This embodiment measures various glucoamylase (EC 3.2.1.3), transglucosidase (EC 2.4.1.24), β-glucosidase (EC 3.2.1.21), α-amylase (EC 3.2.1.1 ) and glucosidase (EC 3.2.1) with the aim of reducing the concentration of leuconostoc and / or oligosaccharide by-products in the concentrated filtrate of the glucan synthesis reaction. Certain enzymes such as DIAZYME RDF ULTRA, transglucosidase (EC 2.4.1.24) and glucoamylase (EC 3.2.1.3), which are alpha-glucosidases, were found to be particularly effective in reducing the amount of these by-products, resulting in There was a corresponding increase in monosaccharides (glucose and fructose) in the treated filtrate.
[0303] According to the procedure outline of Example 1, the filtrate of the dextran synthesis reaction was first prepared and concentrated into a syrup. The composition of the concentrated filtrate is provided in...
Embodiment 3
[0330] Comparison of bond distributions of dextran reaction filtrate fractions before and after enzymatic hydrolysis
[0331] This example measures the effect of transglucosidase (EC 2.4.1.24) and β-glucosidase (EC 3.2.1.21) on the leuconostoc and oligosaccharide by-products present in the concentrated filtrate of the glucan synthesis reaction hydrolytic activity. Transglucosidase was found to reduce the amount of these by-products, resulting in a corresponding increase in the monosaccharides (glucose and fructose) in the treated filtrate.
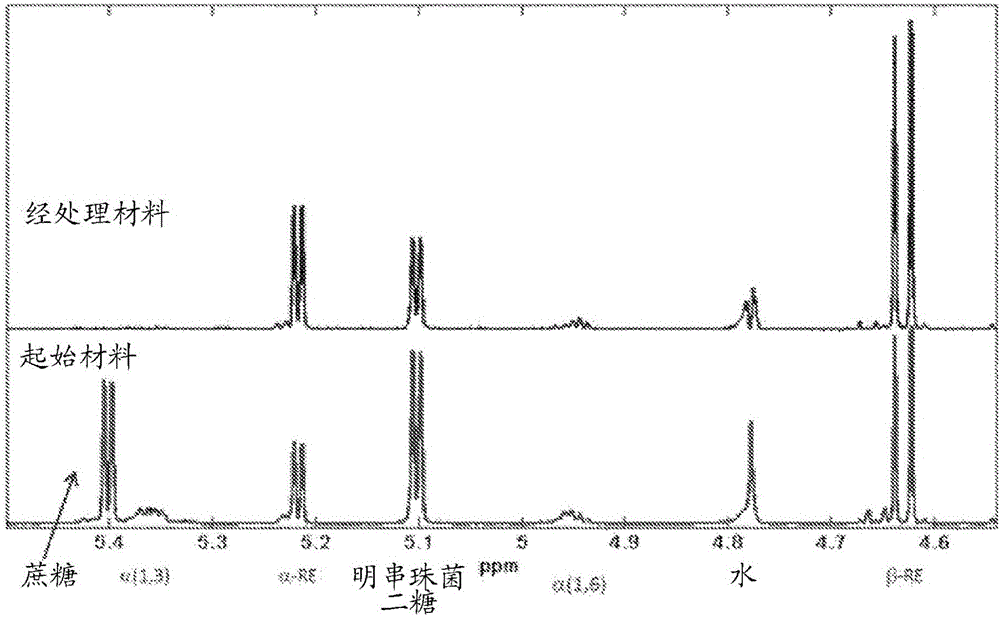
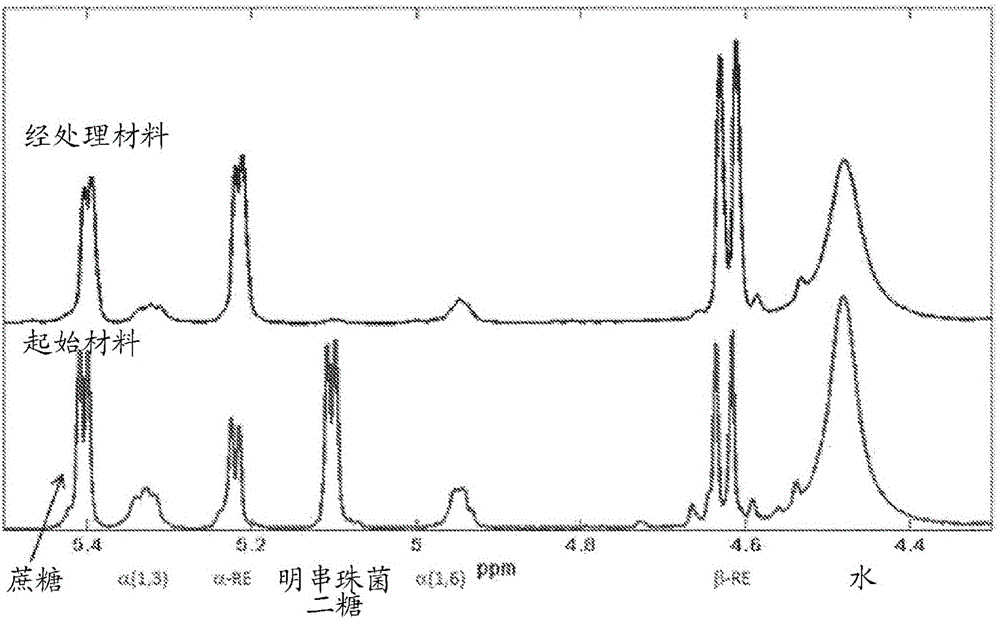
[0332] The oligosaccharide by-products present in the filtrates of the above dextran synthesis reactions contained >90% glucose-glucose linkages as determined by NMR (general method). Of the glucose-glucose bonds, about 78% represent α-1,3 bonds and about 22% represent α-1,6 bonds.
[0333] NMR was used to determine the bond character of the material produced in Example 2.11 above after hydrolysis. Such as figure 1 As shown, the pea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com