Inhibition of osteoclast formation
An osteocytic, neonatal technique for fungal immunomodulatory proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0066] Example 1: Preparation of FIPs
[0067] The FIP-gts of SEQ ID NO: 1 was recombinantly expressed in S. cerevisiae host cells as described in WO2005 / 040375A1. Cells expressing FIP-gts were disrupted, centrifuged, and the supernatant was passed through a filter and molecular sieve to obtain proteins between 10 kDa and 100 kDa. The filtrate was further analyzed by FPLC to 75 column (GE Healthcare) for purification. The purity was over 95% as determined by FPLC.
[0068] FIP-vvo (with SEQ ID NO: 3) and FIP-gmi (with SEQ ID NO: 6) were prepared using the same method described in the present application, and the proteins thus prepared were found to have a purity of over 95%.
example 2
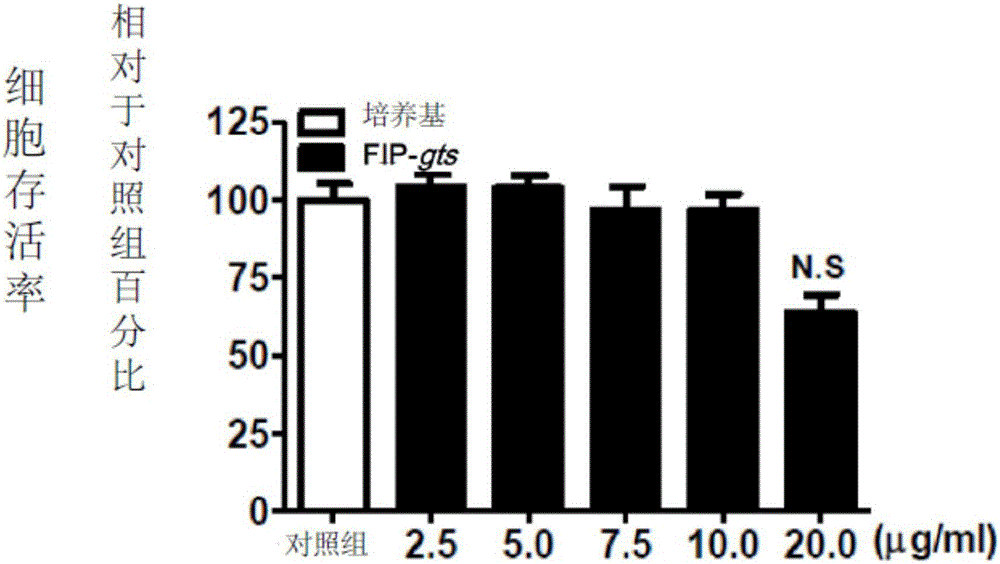
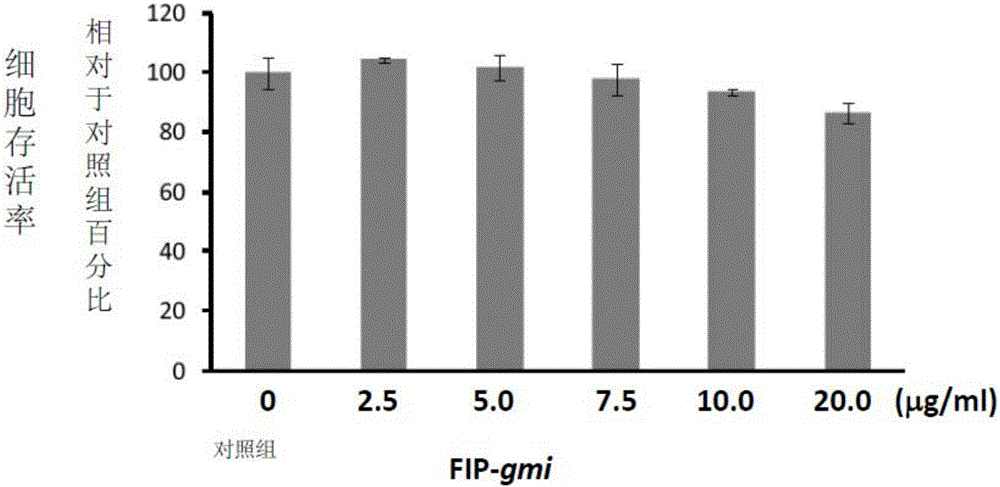
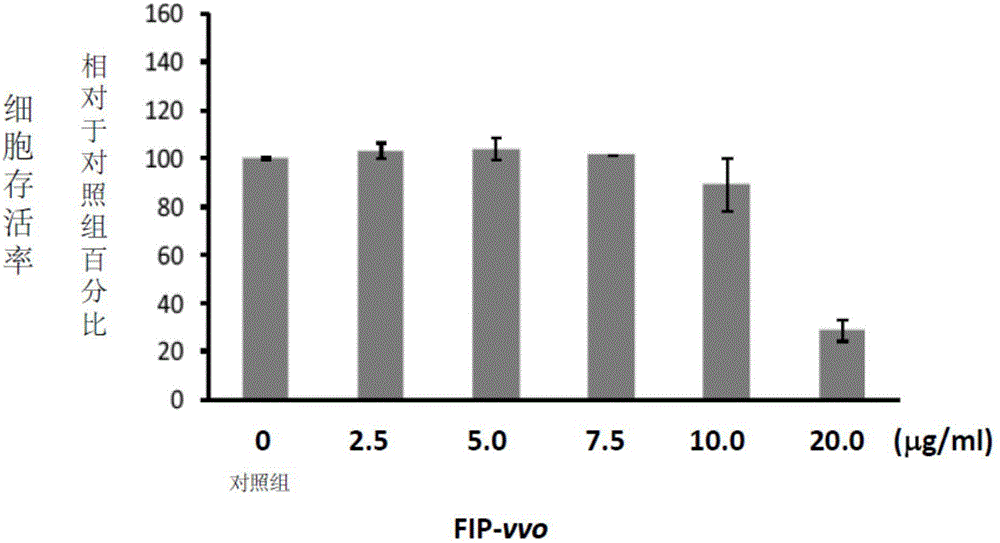
[0069] Example 2: FIP is not toxic to preosteogenic cells
[0070] RAW 264.7 cells, a murine leukemia monocyte / macrophage cell line, were purchased from the Bioresource Conservation and Research Center of the Food Industry Development Institute (Hsinchu, Taiwan). RAW 264.7 cells were divided into 1×10 5 The density of cells / well hole was implanted in 24-well plate culture dish, and in the presence of various concentrations of FIP-gts (2.5-20 μg / ml) prepared in Example 1, cultured in supplemented with Dulbecco's Modified Eagle Medium (DMEM; Sigma Chemical Co., St. Louis, MO, USA) in 10% heat-inactivated calf serum (FBS; Gibco, NY, USA) for 5 days. The supernatant was removed and 25 μl of 2.5 mg / ml MTT (3-[4,5-lutidine-2-yl]-2 , 5-diphenyltetrazolium bromide; Sigma Chemical Co., St. Louis, Missouri, USA). The plates were incubated at 37°C for 1 hour to allow conversion of MTT to water-insoluble formazan crystals. Subsequently, the supernatant was removed and dimethyl sulfoxi...
example 3
[0073] Example 3: Inhibitory Effect of FIP on Osteocytogenesis
[0074] RAW 264.7 cells were suspended in DMEM supplemented with 10% heat-deactivated calf serum, and 1 × 10 5 Cells / well were seeded into 24-well plate culture dishes, and then cultured to the next day. On day 1, the medium was removed and α-minimal basal medium (α-MEM; GIBCO LifeTechnologies, Grand Island, NY, USA) was added to the cell culture with or without 2.5-10 g / ml of FIP-gts and 100 ng / ml of RANKL (PeproTech Inc, Rocky Hill, NJ, USA). On day 5, α-MEM was removed and cell cultures were washed twice with PBS.
[0075] Then, relying on the fact that only mature osteoclasts secrete tartrate-resistant acid phosphatase (TRAP), a TRAP activity assay was used to evaluate the degree of proosteocyte differentiation induced by RANKL. In this assay, cell cultures in each well were treated with 200 microliters of 0.1% Triton X-100, and the cell cultures were washed once with PBS. Next, 100 microliters of TRAP so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com