Purification method of tetracosactide acetate
A technology of ticoctide and purification method, which is applied in the field of polypeptide drug synthesis, can solve the problems of unfavorable large-scale production of ticoctide, complex synthesis process and high production cost, and achieves the improvement of ion pairing ability, the simplification of technological process, and the reduction of Effects of product toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] S1, swelling of 2-Cl-Resin
[0026] Weigh 1g of 2-Cl-Resin and add it into the peptide synthesis reactor from the open end, add DCM solution to the peptide synthesis reactor to immerse 2-Cl-Resin, swell for 30min and drain the solvent;
[0027] Among them, the substitution degree of 2-Cl-Resin is 0.64mmol / g;
[0028] S2, Synthesis of Tecocteptide-2-Cl-Resin
[0029] Tecoctide-2-Cl-Resin is:
[0030] Ser(tBu)-Tyr(tBu)-Se(tBu)r-Met-Glu(OtBu)-His(trt)-Phe-Arg(pbf)-Trp(Boc)-Gly-Lys(Boc)-Pro-Val -Gly-Lys(Boc)-Lys(Boc)-Arg(pbf)-Arg(pbf)-Pro-Val-Lys(Boc)-Val-Tyr(tBu)-Pro-2-Cl-Resin
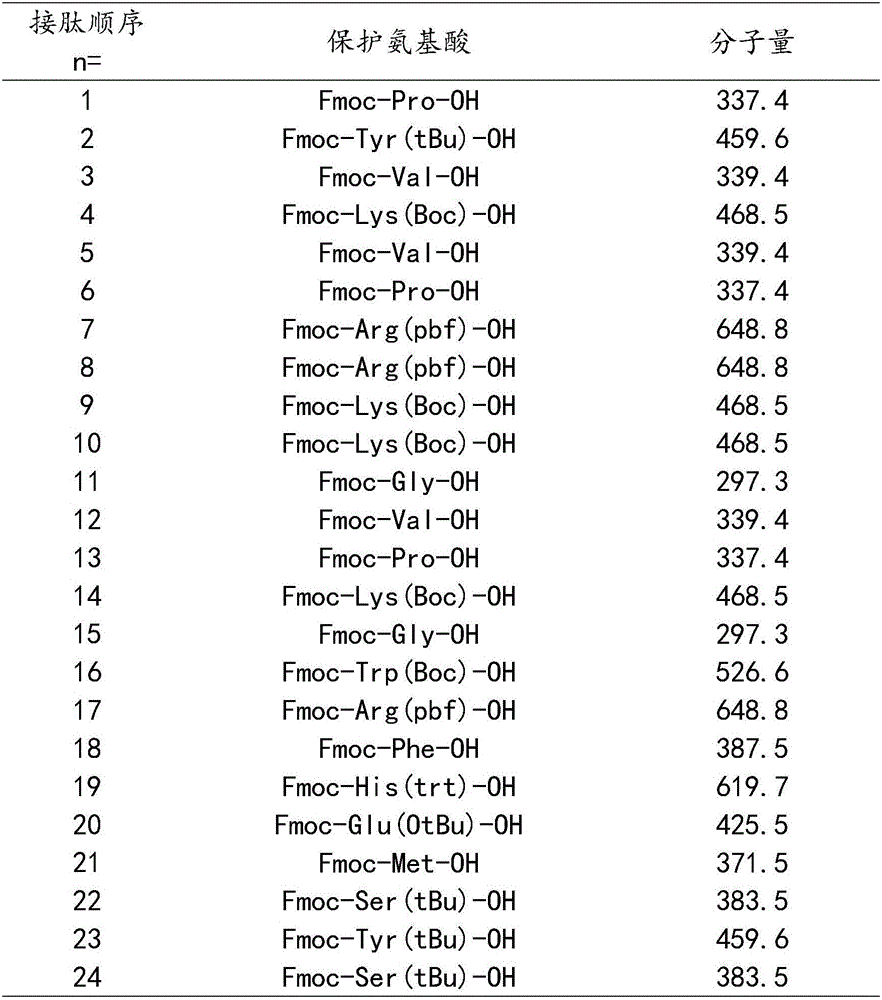
[0031] The protected amino acids used in this example are listed in Table 1 below for the protected amino acids and molecular weights corresponding to the 1-24 amino acids from the resin:
[0032] Table 1
[0033]
[0034] Some commonly used abbreviations in the present invention have the following meanings:
[0035] Fmoc: fluorenylmethoxycarbonyl
[0036] Boc: tert-butoxycarbonyl
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com