Chiral 3,3,5-trimethylcyclohexanone (TMCH) preparation method
A technology of trimethylcyclohexanone and chirality, which is applied in the field of preparation of chiral 3,3,5-trimethylcyclohexanone, and can solve problems such as the research on the reaction mechanism is not very clear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
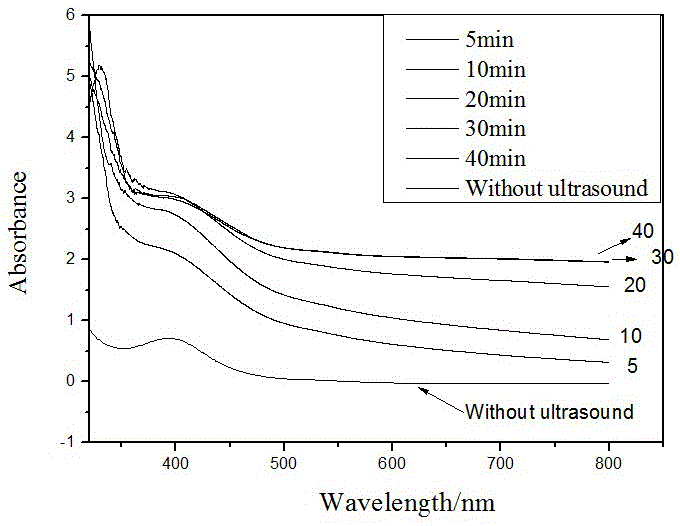
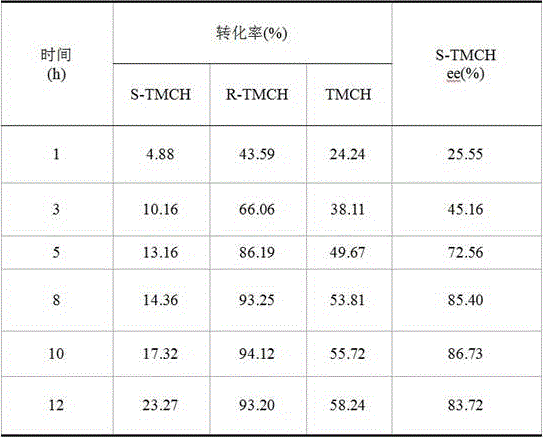
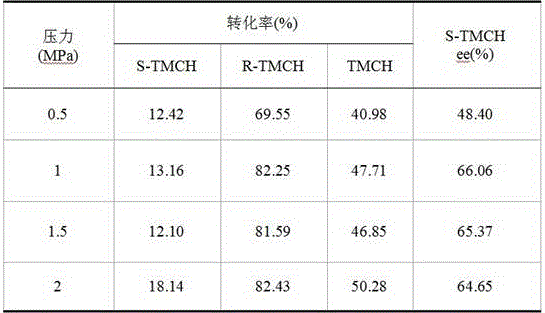
[0020] Preparation of CA-1: Mix palladium acetate, PEG400, and ethanol, and sonicate for 50 minutes to obtain a PEG400-stabilized nano-Pd catalyst. Will Al 2 o 3 The carrier (pseudoboehmite was roasted at 500°C for 4h) was added to the PEG400-stabilized nano-Pd catalyst, stirred for 20min, mixed evenly and sealed for storage. The prepared catalyst and S-proline are not ground, and are directly added into the autoclave during the reaction. This reaction system is recorded as: Pd-Al 2 o 3 +Pro.
[0021] Preparation of CA-2: Mix palladium acetate, PEG400, and ethanol, and sonicate for 50 minutes to obtain a PEG400-stabilized nano-Pd catalyst. Will Al 2 o 3 Carrier (calcined at 500°C for 4h) and S-proline were ground in a mortar for 20min, mixed with PEG400-stabilized nano-Pd catalyst, stirred for 20min, and mixed evenly. This reaction system is recorded as: Pro-Al 2 o 3 -Pd.
[0022] Preparation of CA-3: Mix palladium acetate, PEG400, and ethanol, and sonicate for 50 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com