Synthetic method of alkynyl sulfone derivative
A synthesis method and derivative technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of harsh reaction conditions and the use of strong oxidants, and achieve good substrate applicability, environmental friendliness, and reaction The effect of safe and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
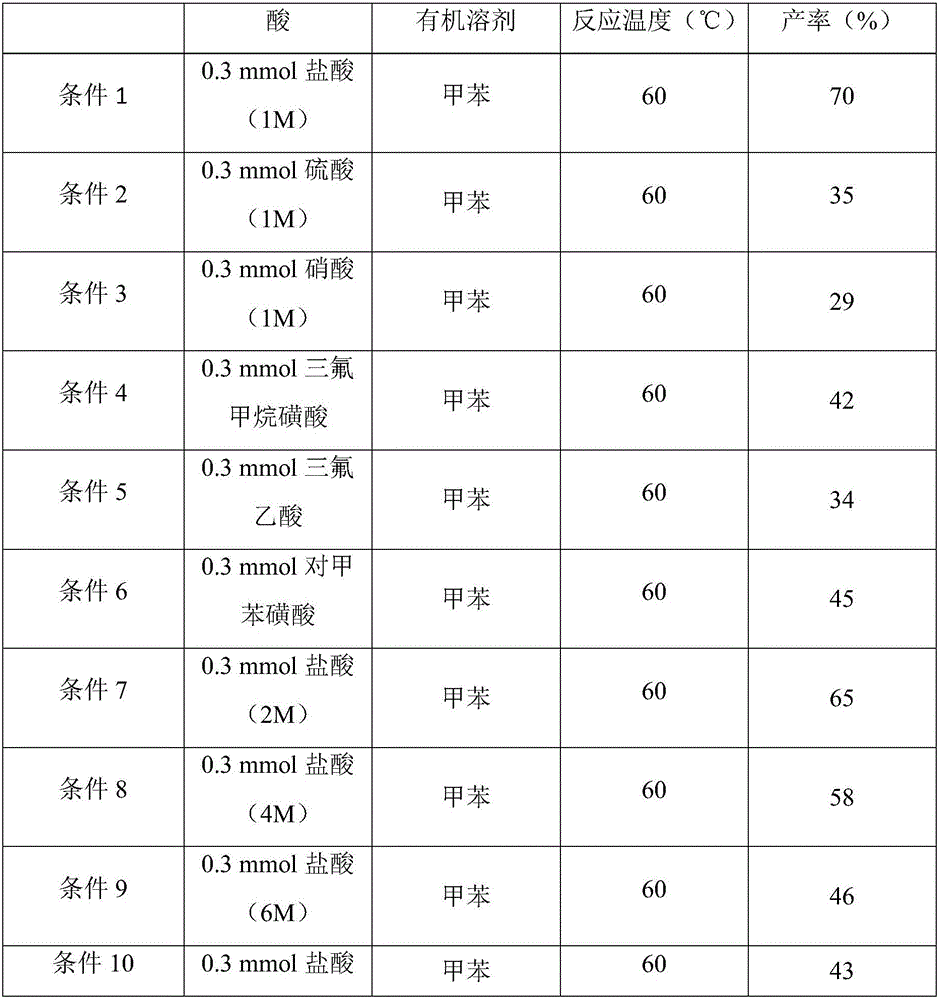
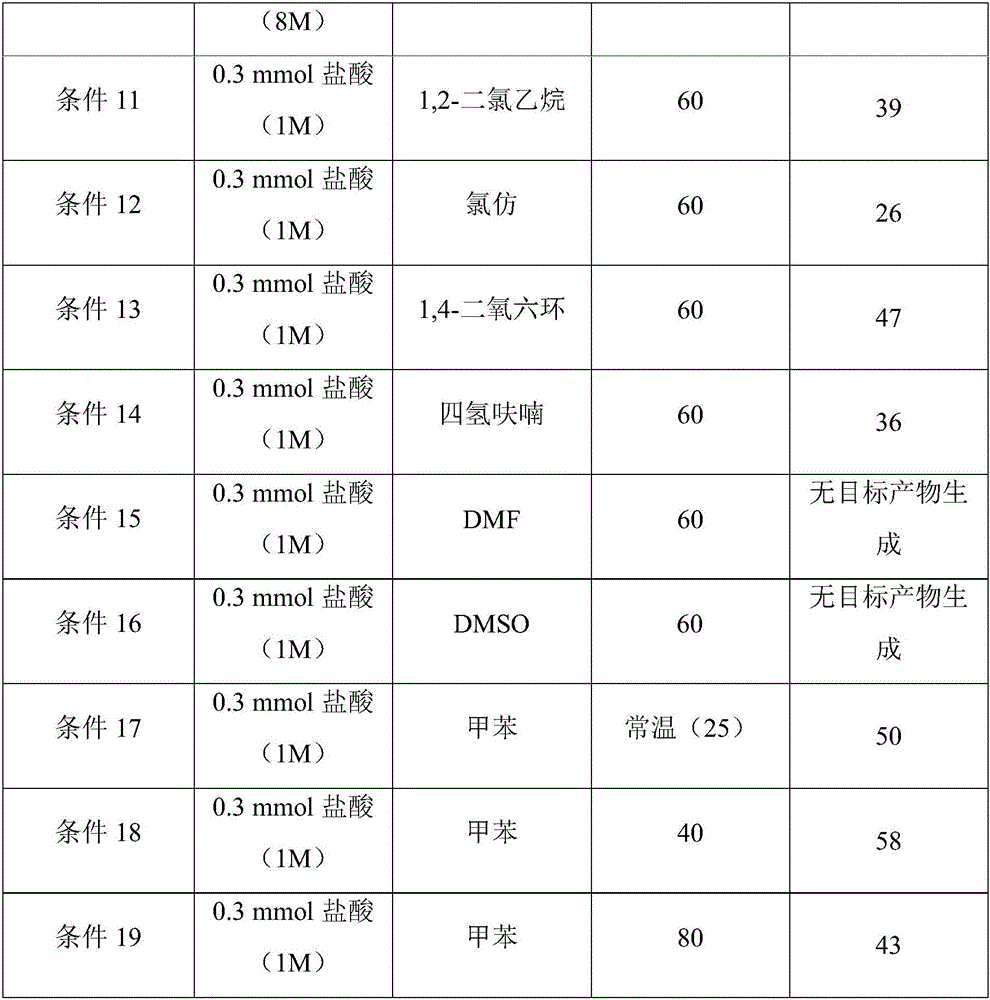
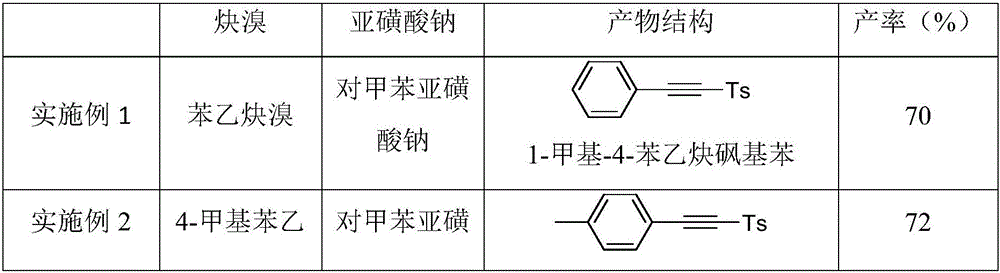
Image
Examples
Embodiment 1
[0033] Example 1: 1 H NMR (400MHz, CDCl 3 )δ7.96(d,J=8.3Hz,2H),7.51(d,J=7.1Hz,2H),7.46–7.44(m,1H),7.40-7.34(m,4H),2.46(s,3H ). 13 C NMR (100MHz, CDCl 3 ) δ 145.3, 138.9, 132.7, 131.4, 130.0, 128.6, 127.4, 118.0, 92.9, 85.6, 21.7.
Embodiment 2
[0034] Example 2: 1 H NMR (400MHz, CDCl 3 )δ7.95(d, J=7.9Hz, 2H), 7.39(t, J=8.7Hz, 4H), 7.16(d, J=7.7Hz, 2H), 2.46(s, 3H), 2.37(s, 3H). 13 C NMR (100MHz, CDCl 3 ) δ 145.2, 142.3, 139.1, 132.7, 129.9, 129.4, 127.4, 114.9, 93.7, 85.2, 21.8, 21.7.
Embodiment 3
[0035] Example 3: 1 H NMR (400MHz, CDCl 3 )δ7.95(d, J=8.0Hz, 2H), 7.43(d, J=7.9Hz, 2H), 7.38(d, J=8.0Hz, 2H), 7.19(d, J=7.9Hz, 2H) ,2.66(q,J=7.6Hz,2H),2.46(s,3H),1.20(t,J=7.6Hz,3H). 13 C NMR (100MHz, CDCl 3 )δ 148.5, 145.4, 139.2, 132.8, 130.0, 128.3, 127.4, 115.1, 93.7, 85.3, 29.0, 21.7, 15.0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com