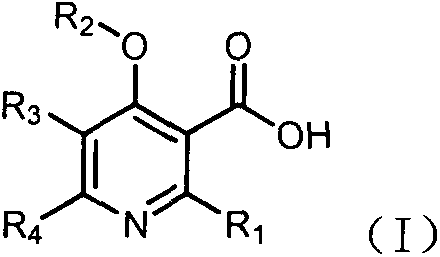
Novel preparation method of 4-alkoxy nicotinic acid compound
A technology for hydrocarbyloxynicotinic acid and hydrocarbyloxynicotinamide, which is applied in the field of preparation of 4-hydrocarboxynicotinic acid compounds, can solve the problems of polychlorinated waste water, expensive and harsh starting materials, etc. The raw materials are simple and easy to obtain, the operation process is easy to control, and the effect of less chlorine-containing waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Synthesis of Niacinamide Compound C
[0038]
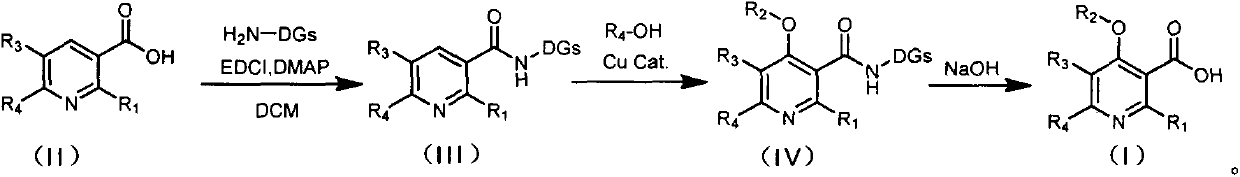
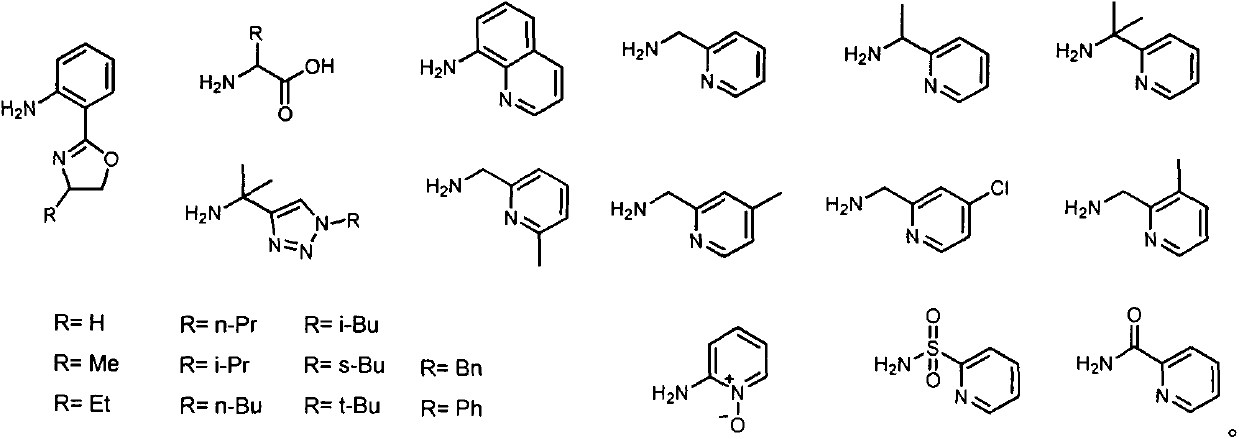
[0039] Weigh nicotinic acid A (1mmol), o-(4,5-dihydro-2-oxazolyl) aniline intermediate B (1mmol), and 4-dimethylaminopyridine DMAP (0.2mmol) in a 50mL eggplant-shaped bottle , dissolved in 5mL of dichloromethane (DCM), added the condensing agent EDCI (1.2mmol) under ice-cooling, stirred at room temperature, tracked and monitored the reaction progress by thin-layer chromatography (TLC), and post-treated for about 6h. Add 10 mL of water to quench the reaction, extract with dichloromethane (15 mL×3) after separation, combine the dichloromethane phases and wash with water (10 mL×2) and saturated sodium chloride solution (10 mL×2) After drying over sodium, the solvent was evaporated under reduced pressure, and after silica gel column chromatography (eluent: petroleum ether / ethyl acetate = 10:1), the nicotinamide compound C was obtained with a yield of 74%. LC-MS (ESI+) m / z: Calcd.for [M+H: C 15 h 14 N 3 o 2 ]:...
Embodiment 2
[0040] Example 2: Synthesis of 4-alkoxynicotinamide compound D
[0041]
[0042] Weigh nicotinamide compound C (1mmol), sodium carbonate (2mmol) and copper acetate (0.5mmol) into the reaction flask in turn, add DMSO (5mL) to it, and then add 3-methylphenol (3mmol), React at 80°C for 8 hours, cool to room temperature, add ethyl acetate (EtOAc, 20mL) to dilute, then add (28%-30%) ammonium hydroxide solution (NH 3 ·H 2 (20, 8 mL) into the reaction system, continue stirring for 20 min, extract with ethyl acetate (EtOAc, 20 mL×3), combine the organic phases and wash with saturated sodium bicarbonate solution (NaHCO 3 , 10mL×3), washed with water (10mL×3), washed with saturated sodium chloride (NaCl, 10mL×3) solution, and washed the organic phase with anhydrous sodium sulfate (NaCl 2 SO 4 ) drying, after concentration, carry out column chromatography (V 石油醚 :V 乙酸乙酯 =12:1), a white solid D was obtained with a yield of 83%.
[0043] LC-MS (ESI+) m / z: Calcd.for [M+H: C 22 h ...
Embodiment 3
[0046] Example 3: Synthesis of 4-alkoxynicotinic acid compound E
[0047]
[0048] Weigh 2-(3-trifluoromethyl)phenoxynicotinamide compound D (1mmol) and sodium hydroxide (40mmol) into a reaction flask, add 5mL of ethanol, and react at 80°C for 6h. After the reaction is complete, The organic solvent was evaporated to dryness, water was added, and extracted with ethyl acetate (EtOAc) (15mL×3), the organic phases were combined and dried by adding anhydrous sodium sulfate, concentrated and then column chromatographed (eluent: V 石油醚 :V 乙酸乙酯 =20:1), and 125 mg of o-(4,5-dihydro-2-oxazolyl)aniline intermediate B was recovered with a yield of 77.6%. Add 1M hydrochloric acid (HCl) solution to the aqueous phase, adjust the pH to 4, extract with ethyl acetate (EtOAc) (15mL×3), add anhydrous sodium sulfate to dry, concentrate and column chromatography (eluent: V 石油醚 :V 乙酸乙酯 =2:1) to obtain 4-(3-methyl)-phenoxynicotinic acid E with a yield of 77%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com