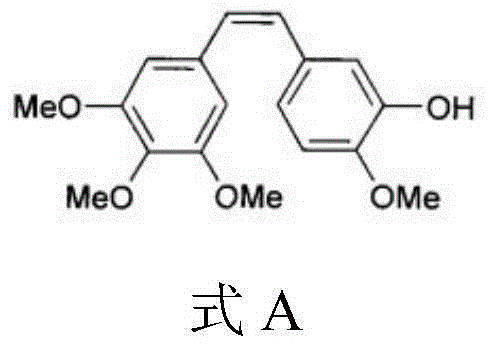
Combretastatin A4 derivative and preparation thereof
A technology of combretastatin and derivatives, applied in the field of combretastatin A4 derivatives and preparations thereof, to achieve the effects of solving poor lipid solubility, high drug concentration and improving chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
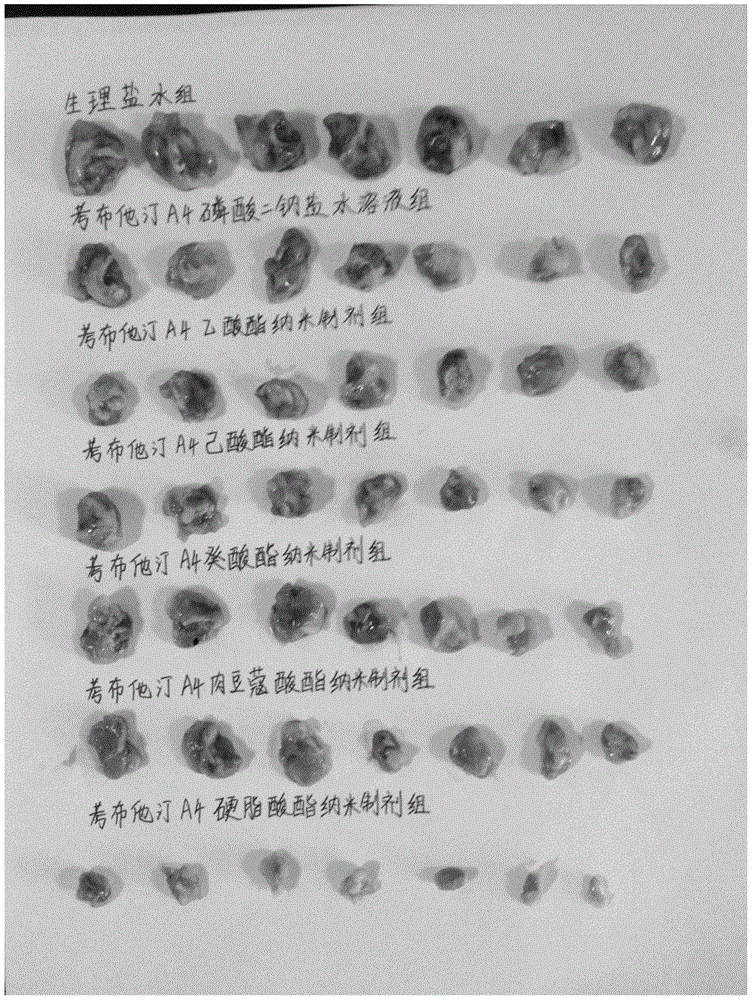
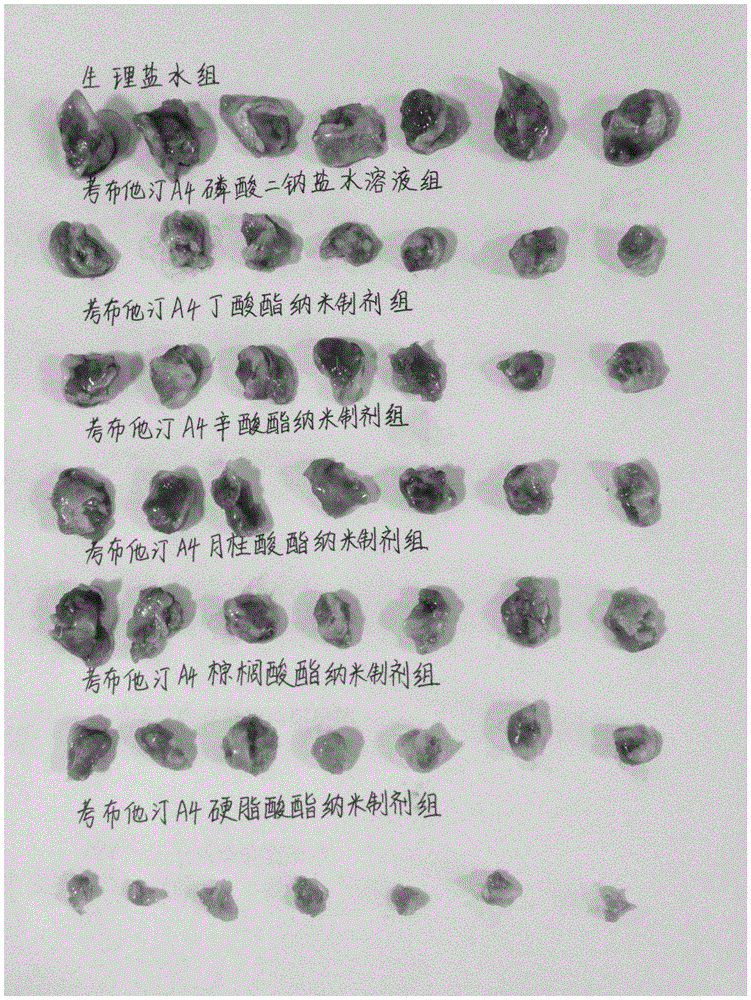
[0056] Example 1: Comparative evaluation of series of different combretastatin A4 fatty acid esters in vivo and in vitro
[0057] The international patent (WO 2007059118 A1) records that the carbon chain length of fatty acids is in the range of C2-C21, basically covering all common fatty acids, but does not clearly record the corresponding substantial effects. For this reason, in a parallel comparative experiment, we randomly designed and synthesized 10 combretastatin A4 esters with different fatty acid carbon chains within this range, that is, combretastatin A4 acetate, butyrate, hexanoate, caprylate , caprate, laurate, myristate, palmitate, stearate, oleate, including combretastatin A4 esters of saturated fatty acids and unsaturated fatty acids, which were evaluated in vivo and in vitro.
[0058] (1) Preparation of a series of different combretastatin A4 fatty acid esters
[0059] 1. Preparation of combretastatin A4 acetate
[0060] Add 250 mg of combretastatin A4 to the r...
Embodiment 2
[0159] Example 2: Comparative study on the preparation process of combretastatin A4 stearate nano-preparation injection
[0160]Generally, there are three methods for preparing nano-preparations for injection, namely, melting high-pressure homogenization method, thin-film high-pressure homogenization method and injection high-pressure homogenization method. feasibility study.
[0161] (1) Melting high pressure homogenization method
[0162] The melting high-pressure homogenization method is to mix the drug or the drug with the lipid material, make it in a molten or semi-molten state at a certain temperature, emulsify it in water under the action of a surfactant, and then further reduce it through a high-pressure homogenizer. particle size to obtain nano-preparations. According to its principle, we use this method to prepare combretastatin A4 stearate nano-preparation with the most conservative drug loading of 0.5mg / ml, and conduct quality evaluation.
[0163] 1. Preparation...
Embodiment 3
[0186] Example 3: Research on the preparation process of combretastatin A4 stearate nano-preparation injection
[0187] A large number of tests have shown that the commonly used nano-preparation method is not suitable for the preparation of combretastatin A4 stearate nano-preparation. But in comparison, the nanoparticles prepared by injecting high-pressure homogeneous method are relatively the best. In order to investigate the reason, we repeatedly conducted decomposition tests on injection high-pressure homogenization method, and unexpectedly found that a relatively good nano-preparation has been formed at the moment when the lipid solution is injected into water for injection, and then it is processed by conventional thinking. On the contrary, the high-pressure homogenization will make the distribution of nanoparticles uneven, and even cause damage to the nanoparticles.
[0188] Regarding this conclusion, we have carried out many comparative experiments to confirm it, and a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com