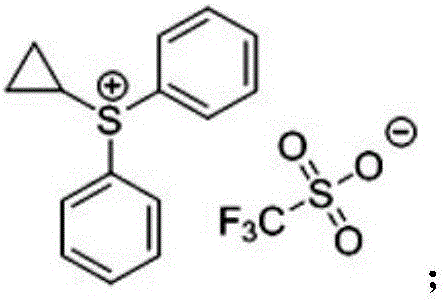
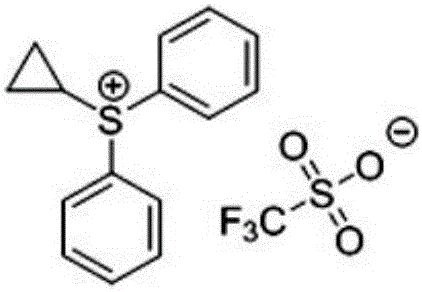
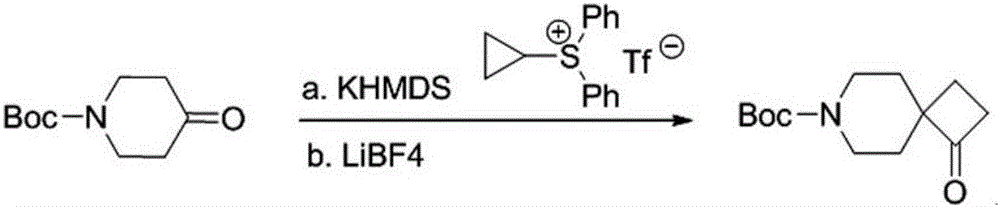
Use of cyclopropyl diphenylsulfonium trifluoromethanesulfonate as sulfur ylide reagent and method for preparation of four-membered cyclic ketone
A technology of cyclopropyldiphenylsulfonium and trifluoromethanesulfonate, which is applied in the field of cyclopropyldiphenylsulfonium trifluoromethanesulfonate, can solve the problems of difficult synthesis, high price, complicated operation, etc. Achieve the effect of solving the difficulty of synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Dissolve 30 g (0.083 mol) of cyclopropyldiphenylsulfonium trifluoromethanesulfonate in 300 mL of anhydrous tetrahydrofuran, cool to -40 ° C, and then add 91 mL of bis(trimethylsilyl ) Potassium Amide; after the dropwise addition, continue to stir for 5 minutes, then add 16.6g (0.083mol) 4-Boc-piperidone to the reaction system at one time, react at -40°C for 20 minutes, naturally warm to room temperature and stir for 3 hours. After the reaction was completed, 300 mL of water was added to quench the reaction, extracted three times with diethyl ether (200 mL×3) and the organic phases were combined, dried with anhydrous sodium sulfate, and concentrated to obtain an oil. Dissolve the obtained oil in 300 mL of toluene, add 7.78 g (0.083 mol) of lithium tetrafluoroborate, react at 80 ° C for 2 h, cool to room temperature, remove toluene under reduced pressure, add 300 mL of water to quench the reaction, and extract three times with ether (200 mL× 3) Combine the organic phases,...
Embodiment 2
[0024] 10g (0.10mol) tetrahydropyrone and 39.6g (0.11mol) cyclopropyldiphenylsulfonium trifluoromethanesulfonate were dissolved in 200mL DMSO, and 11.2g ( 0.20mol) KOH, naturally rose to room temperature and reacted for 8h. After the reaction was completed, 300 mL of saturated ammonium chloride solution was added to quench the reaction, and stirring was continued overnight. Extracted three times with ethyl acetate (150 mL×2) and combined the organic phases, washed the organic phase twice with saturated brine (75 mL×2), dried over anhydrous sodium sulfate, and concentrated to obtain a crude product. The obtained crude product was separated by a silica gel column to obtain 11.2 g of the product with a yield of 80%.
[0025] The reaction formula of this reaction is as follows:
[0026]
Embodiment 3
[0028] 14g (0.10mol) of p-chlorobenzaldehyde and 35.9g (0.10mol) of cyclopropyldiphenylsulfonium trifluoromethanesulfonate were dissolved in 250mL of anhydrous tetrahydrofuran, and 15.7g (0.14 mol) tetrahydrofuran solution of potassium tert-butoxide. After the dropwise addition, it was naturally raised to room temperature. After continuing to react for 3 hours, 1M tetrafluoroboric acid aqueous solution was added, and stirring was continued at room temperature for 3 hours. After the reaction was completed, 300 mL of diethyl ether was added to the reaction solution to extract the organic phase, and then the organic phase was washed with saturated sodium bicarbonate and saturated brine respectively, and dried with anhydrous sodium sulfate to obtain a crude product. The obtained crude product was separated by a silica gel column to obtain 15.3 g of the product with a yield of 85%.
[0029] The reaction formula of this reaction is as follows:
[0030]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com