Preparation method for Stivarga midbody of medicine for treating cancer
A technology of regorafenib and intermediates, applied in the field of medicinal chemical preparation, can solve problems such as low yield, complicated reaction process, and difficult reaction treatment, and achieve the effects of simple operation, reduced side reactions, and easy industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
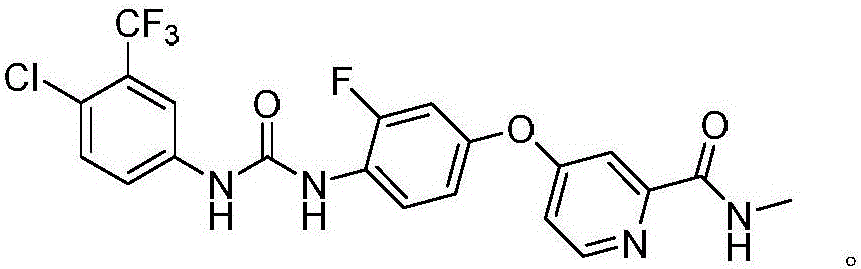
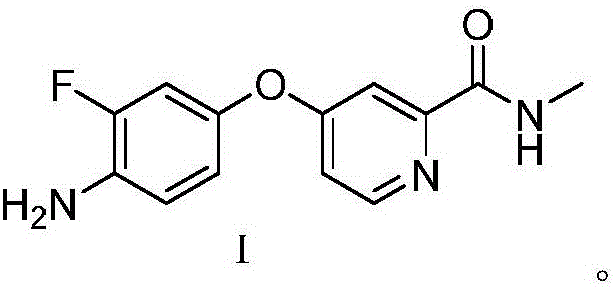
[0021] Preparation of regorafenib intermediate 4-(4-amino-3-trifluoromethyl)-N-methylpyridine-2-carboxamide
[0022] In a three-necked flask, add 12.7g of 3-fluoro-4-aminophenol, 0.95g of cuprous iodide, and 63.7g of potassium phosphate, start stirring and heat to 85°C, and then add 4-chloro-2-pyridinecarboxamide in three batches Add 18.6g of hydrochloride into the reaction system, keep the temperature and continue the reaction for 6 hours, contact reaction in N,N-dimethylformamide to obtain regorafenib intermediate 4-(4-amino-3-trifluoromethyl) -21.4 g of N-methylpyridine-2-carboxamide, yield 91.1%. MS(ESI):m / z[M+H] + 262.10.
[0023] 1 HNMR (400MHz, d 6 -DMSO)δ: 8.81(q,1H), 8.50(d,1H), 7.41(d,1H), 7.13(dd,1H), 7.07(dd,1H), 6.97(t,1H), 6.87(dd ,1H), 5.50(brs,2H), 2.80(d,3H). .
Embodiment 2
[0025] Preparation of regorafenib intermediate 4-(4-amino-3-trifluoromethyl)-N-methylpyridine-2-carboxamide
[0026] In a three-necked flask, add 12.7g of 3-fluoro-4-aminophenol, 1.9g of cuprous iodide, and 106.1g of potassium phosphate, start stirring and heat to 90°C, and then add 4-chloro-2-pyridinecarboxamide in three batches Add 18.6g of hydrochloride into the reaction system, keep the temperature and continue the reaction for 5 hours, contact reaction in N,N-dimethylformamide to obtain regorafenib intermediate 4-(4-amino-3-trifluoromethyl) -21.2 g of N-methylpyridine-2-carboxamide, yield 90.3%.
Embodiment 3
[0028] Preparation of regorafenib intermediate 4-(4-amino-3-trifluoromethyl)-N-methylpyridine-2-carboxamide
[0029] In a three-necked flask, add 12.7g of 3-fluoro-4-aminophenol, 0.95g of cuprous iodide, and 55.2g of potassium carbonate, start stirring and heat to 100°C, and then add 4-chloro-2-pyridinecarboxamide in three batches Add 20.7g of hydrochloride into the reaction system, keep the temperature and continue the reaction for 7 hours, contact reaction in N,N-dimethylformamide to obtain regorafenib intermediate 4-(4-amino-3-trifluoromethyl) -23.5 g of N-methylpyridine-2-carboxamide, yield 89.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com