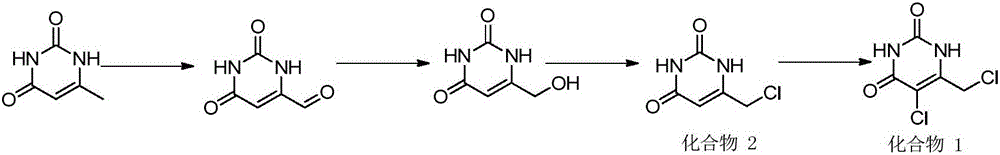
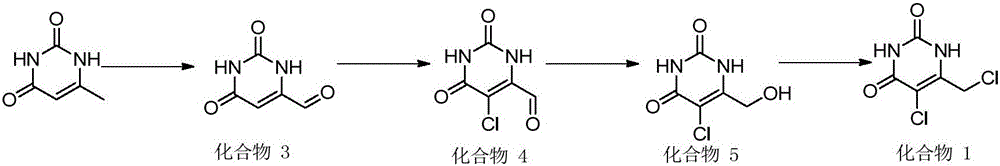
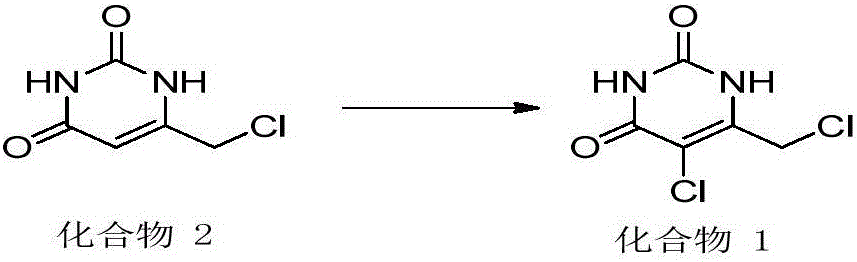
5-chloro-6-(chloromethyl) uracil and preparation method thereof
A technology of chloromethyl and uracil, applied in the field of 5-chloro-6-uracil and its preparation, can solve the problems of incomplete chlorination, long synthesis route and high synthesis cost, and achieves complete chlorination reaction and high cost Low, method-reliable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The method for preparing 5-chloro-6-(chloromethyl)uracil provided in this embodiment specifically comprises the following steps:
[0045] (1) At 0°C, add 100g of ethyl 4-chloroacetoacetate and 500ml of dichloromethane to the three-necked flask in turn, stir at 0°C, then slowly add 86.17g of sulfuryl chloride dropwise, keeping the temperature at 0-5°C , reacted for 8 hours, the reaction was completed, concentrated, and distilled under reduced pressure to obtain 75 grams of ethyl 2,4-dichloroacetoacetate;
[0046](2), at room temperature, sequentially add 10 grams of urea, 33.17 grams of ethyl 2,4-dichloroacetoacetate and 200 grams of polyphosphoric acid in the three-necked bottle in step (2), start the mechanical stirring, and heat up to 100 ° C, React for 16 hours, after the reaction is complete, cool down to 50°C, add 200ml of water, drop to 20-30°C, filter, rinse the filter cake with ethyl acetate, and dry to obtain light brown solid 5-chloro-6-(chloromethyl ) Uracil...
Embodiment 2
[0048] The method for preparing 5-chloro-6-(chloromethyl)uracil provided in this embodiment specifically comprises the following steps:
[0049] (1), with the step (1) in the embodiment;
[0050] (2), at room temperature, sequentially add 10 grams of urea, 33.17 grams of ethyl 2,4-dichloroacetoacetate and 200 grams of polyphosphoric acid in the three-necked bottle in step (2), start the mechanical stirring, and heat up to 100 ° C, React for 14 hours, after the reaction is complete, cool down to 50°C, add 200ml of water, drop to 20-30°C, filter, rinse the filter cake with ethyl acetate, and dry to obtain light brown solid 5-chloro-6-(chloromethyl ) uracil 21 grams.
Embodiment 3
[0052] The method for preparing 5-chloro-6-(chloromethyl)uracil provided in this embodiment specifically comprises the following steps:
[0053] (1), with the step (1) in the embodiment;
[0054] (2), at room temperature, sequentially add 10 grams of urea, 33.17 grams of ethyl 2,4-dichloroacetoacetate and 200 grams of polyphosphoric acid in the three-necked bottle in step (2), start the mechanical stirring, and heat up to 90 ° C, React for 16 hours, after the reaction is complete, cool down to 50°C, add 200ml of water, drop to 20-30°C, filter, rinse the filter cake with ethyl acetate, and dry to obtain light brown solid 5-chloro-6-(chloromethyl ) 19.5 grams of uracil.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com