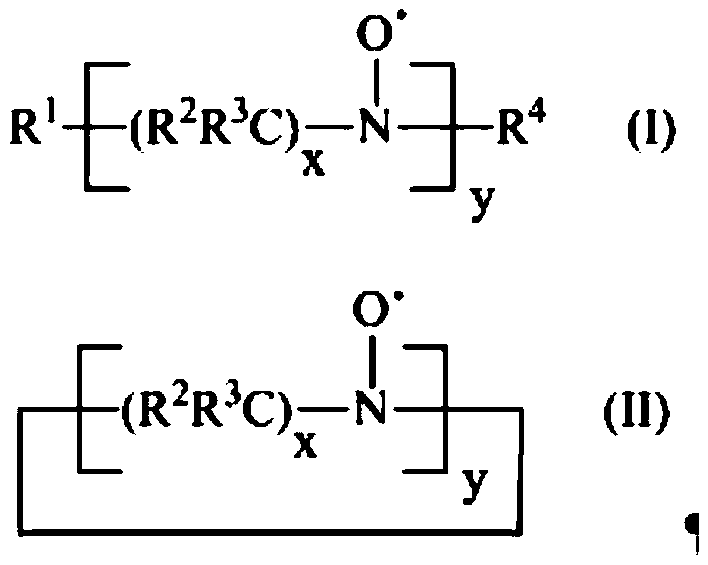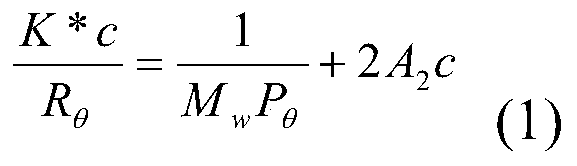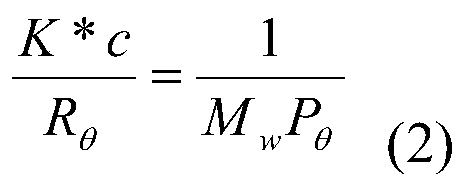Process for the preparation of diene polymers or random vinylarene-diene copolymers
A technology of vinyl aromatic hydrocarbon and diene copolymer, applied in the field of preparing diene polymer or random vinyl aromatic hydrocarbon-diene copolymer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Embodiment 1 (comparison)
[0101] 600 grams of anhydrous cyclohexane (Bitolea), followed by 27 grams of freshly distilled anhydrous 1,3-butadiene (Versalis SpA) were introduced into a 1 liter vessel equipped with a jacket for circulation of a temperature-controlled fluid. in a stirred reactor. The reactor was equipped with a system for continuous measurement of the UV-Vis absorption spectrum of the polymer solution, which functioned as described in Example 5. The temperature of the reaction mixture was thermostatically controlled to 80°C and kept constant within ±4°C throughout the duration of the test. Then, 0.5 mmol of n-butyllithium (Aldrich) were introduced: the reaction conditions were maintained for 60 minutes, at the end ethanol (Carlo Erba) was introduced in an equimolecular amount relative to the amount of n-butyllithium introduced. Then, the obtained polymer solution was discharged from the reactor, and a phenolic antioxidant (from Ciba 1520, in an amou...
Embodiment 2
[0103] Embodiment 2 (comparison)
[0104] 600 grams of anhydrous cyclohexane (Bitolea), followed by 27 grams of freshly distilled anhydrous 1,3-butadiene (Versalis SpA) were introduced into a 1 liter vessel equipped with a jacket for circulation of a temperature-controlled fluid. in a stirred reactor. The reactor was equipped with a system for continuous measurement of the UV-Vis absorption spectrum of the polymer solution, which functioned as described in Example 5. The temperature of the reaction mixture was thermostatically controlled to 120°C and kept constant within ±4°C throughout the duration of the test. Then, 0.5 mmol of n-butyllithium (Aldrich) were introduced: the reaction conditions were maintained for 60 minutes, at the end ethanol (Carlo Erba) was introduced in an equimolecular amount relative to the amount of n-butyllithium introduced. Then, the obtained polymer solution was discharged from the reactor, and a phenolic antioxidant (from Ciba 1520, in an amoun...
Embodiment 3
[0106] Embodiment 3 (invention)
[0107] 600 g of anhydrous cyclohexane (Bitolea), followed by 27 g of freshly distilled anhydrous 1,3-butadiene (Versalis SpA) and then obtained as described in US patent application US 2010 / 0240909 0.5 mmol of 1,1,3,3-tetraethylisoindol-2-yl-oxyl (TEDIO) was introduced into a 1 liter stirred reactor equipped with a jacket for circulation of a temperature-controlled fluid. The reactor was equipped with a system for continuous measurement of the UV-Vis absorption spectrum of the polymer solution, which functioned as described in Example 5. The temperature of the reaction mixture was thermostatically controlled to 120°C and kept constant within ±4°C throughout the duration of the test. Then, 0.5 mmol of n-butyllithium (Aldrich) was introduced to obtain about 1:1 ratio of 1,1,3,3-tetraethylisoindol-2-yl-oxyl (TEDIO) and active n-butyllithium Molar ratio between the amounts: The reaction conditions were maintained for 60 minutes, at the end of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com