Quercetin derivative and preparation method and application thereof
A derivative, quercetin technology, applied in the field of medicine, can solve problems such as poor fat solubility, low drug efficacy, and limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
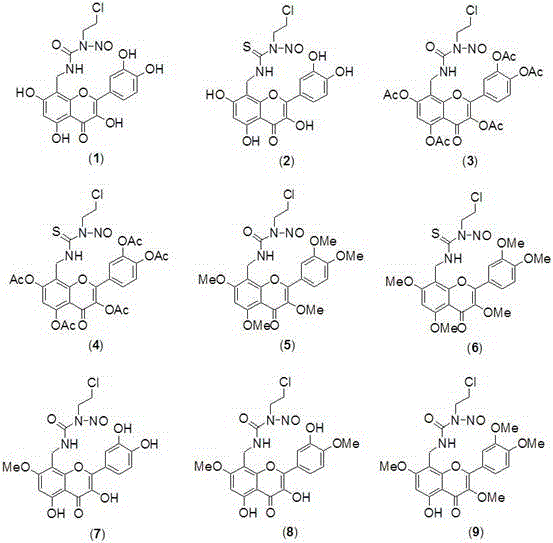
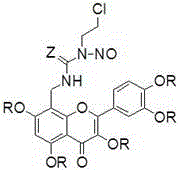
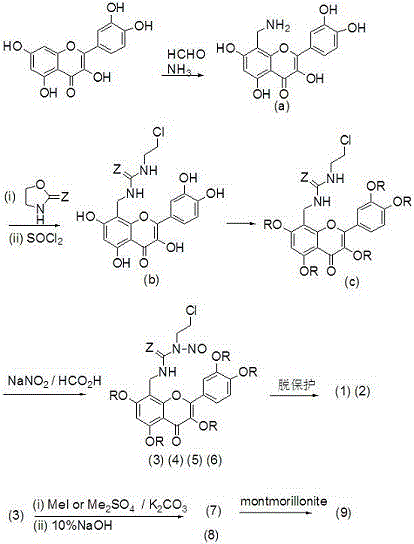
[0020] Preparation of Quercetin Derivatives (1) and (5)
[0021] Add 122 mg (1.5 mmol) of formaldehyde solution, 175 mg (1.5 mmol) of concentrated ammonia water, 302 mg (1 mmol) of quercetin and 5 mL of ethanol into a closed pressure-resistant reactor, add 3 drops of hydrochloric acid, and heat to 75- React at 80°C for 3 hours, cool naturally, precipitate a solid, filter with suction, and separate the crude product by chromatography (V (n-butanol): V (water): V (HOAc) = 4:1:1, eluted with methanol to obtain intermediate Body (a) 105 mg light yellow solid, yield 31.7%. m.p.>300℃; 1 H NMR (400 MHz, DMSO-d 6 )δ: 7.74 (d,1H), 7.60 (d, 1H), 6.92 (d, 1H), 6.19 (s, 1H), 3.86 (s, 2H).
[0022] N 2 Under protection, 331 mg (1.0 mmol) of intermediate (a) and 130.5 mg (1.5 mmol) of 2-oxazolidinone were mixed, heated to 120°C for 2 hours, cooled to 0°C, and 141.6 mg (1.2 mmol) of SOCl was added 2 , heated to 100°C for 2 hours, cooled, added 15 mL of water, precipitated solid, filtered...
Embodiment 2
[0027] Preparation of Quercetin Derivatives (2) and (6)
[0028] 154.5 mg (1.5 mmol) 2-oxazolethione was used to replace 130.5 mg (1.5 mmol) 2-oxazolidinone in Example 1, and other operations were the same as in Example 1 to obtain 190.7 mg of compound (2) yellow solid, Compound (6) was obtained as a yellow solid 197.9 mg, m.p. were >300°C; compound (2) 1 H NMR (400 MHz, DMSO-d 6 )δ: 7.73 (d, 1H), 7.59 (d,1H), 6.89 (d, 1H), 6.79 (s, 1H), 6.18 (s, 1H), 4.24-4.10 (t, 2H), 3.88 (s ,2H), 3.87-3.80 (m, 1H). EIS [M-Na] + = 458.0. Compound (6) 1 H NMR (400 MHz, DMSO-d 6 )δ: 7.72 (d, 1H), 7.59 (d, 1H), 6.88 (d, 1H), 6.79 (s, 1H), 6.17 (s, 1H),4.24-4.10 (t, 2H), 3.87 (s , 2H), 3.87-3.80 (m, 1H), 3.71 (s, 15H). EIS [M+Na] + = 574.0.
Embodiment 3
[0030] Preparation of Quercetin Derivatives (3)
[0031] Replace 759 mg (5.5 mmol) of K in Example 1 with 300 mg (8.1 mmol) of anhydrous NaOAc and 7 mL of acetic anhydride 2 CO 3 and 781 mg (5.5 mmol) CH 3 I, reflux reaction for 2 hours, after the reaction was completed, poured into 100 mL of ice water, filtered, washed with water until neutral, dried, recrystallized from 95% ethanol, other operations were the same as in Example 1, and 230.9 mg of compound (3) was obtained as a yellow solid, m.p.> 300°C;1 H NMR (400 MHz, DMSO-d 6 )δ: 7.73 (d, 1H), 7.59 (d, 1H), 6.89 (d, 1H), 6.79 (s, 1H), 6.18 (s, 1H), 4.24-4.10 (t, 2H), 3.88 (s , 2H), 3.87-3.80 (m,1H), 2.04 (s, 15H). EIS [M+Na] + = 698.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com