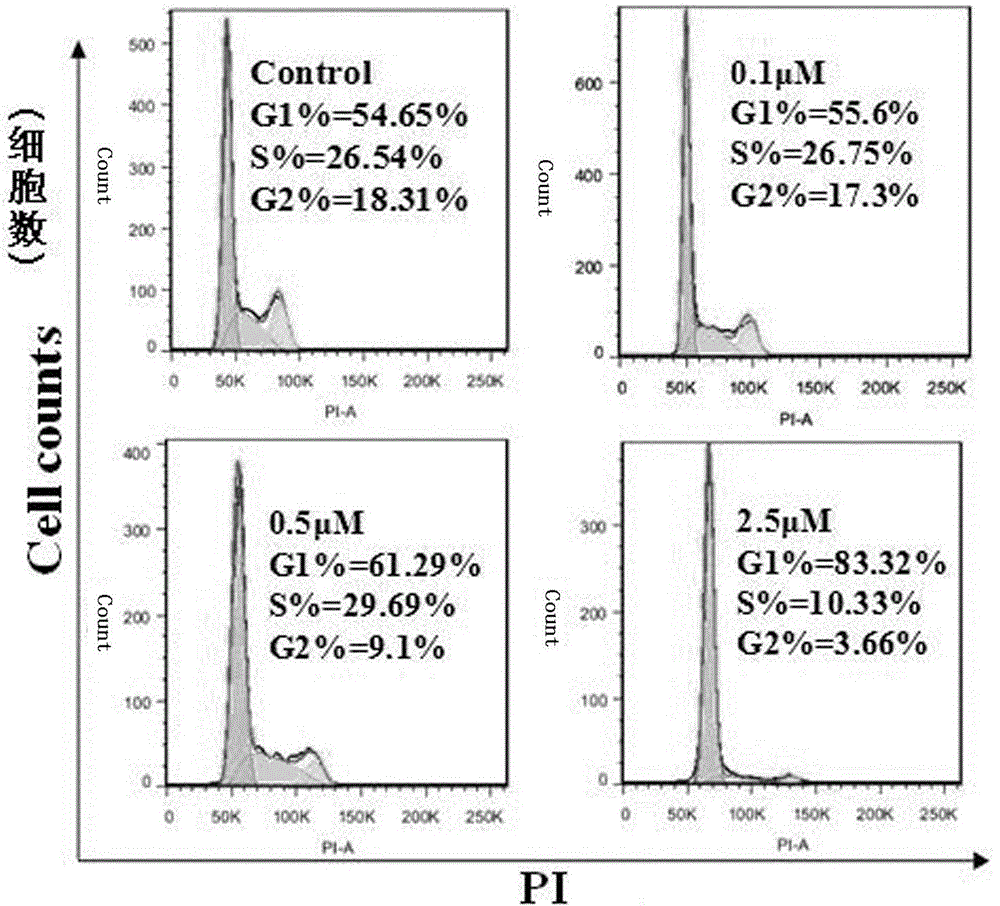
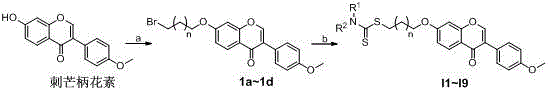
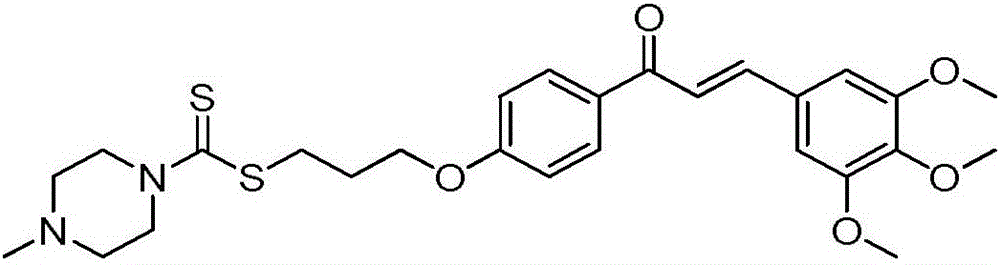
Formononetin derivative comprising dithiocarbamate, preparation method and application thereof to antitumor drugs
A technology containing carbamodithiocarbamate and formononetin, which is applied in the fields of antineoplastic drugs, drug combinations, organic chemistry, etc., can solve the problems of weak antitumor activity, improve antitumor activity, and solve the problem of antitumor activity. The effect of weak tumor activity and widening the field of research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation of embodiment 1 general formula (1a~1d)
[0028] In 100ml tetrahydrofuran, react 10g formononetin, 6.2g potassium carbonate and 7g 1,2-dibromoethane or 7.5g 1,3-dibromopropane or 8g 1,4-dibromobutane at 80°C Heat and stir. After reacting for 6 hours, the system was concentrated to dryness, and 10 ml of ethanol was added for recrystallization to obtain compounds 1a-1d.
Embodiment 2
[0029] The preparation of embodiment 2 general formula (I1~I9)
[0030] In 10ml of acetone, react 1g of compound (1a~1d) with 600mg of substituted secondary amine, 300mg of carbon disulfide, and 440mg of sodium phosphate at room temperature. After the reaction is completed, filter with suction, concentrate the filtrate, and recrystallize from 3ml of ethanol to obtain the general formula (I1~ I9) Compounds.
[0031] The general formula (I1~I9) structure is characterized as follows:
[0032] I-1: Yield 83%. White solid. Mp: 124~125℃. 1 H NMR (400MHz, DMSO) δ8.42 (s, 1H), 8.03 (d, J = 8.9Hz, 1H), 7.53 (d, J = 8.7Hz, 2H), 7.38–7.29 (m, 4H), 7.29 –7.22(m,1H),7.17(d,J=2.2Hz,1H),7.09(dd,J=8.9,2.3Hz,1H),7.00(d,J=8.8Hz,2H),4.22(t, J=6.1Hz, 4H), 3.92(s, 2H), 3.79(s, 3H), 3.51(s, 2H), 3.42(t, J=7.2Hz, 2H), 2.45(s, 4H), 2.22– 2.09(m,2H). 13 C NMR(100MHz,DMSO)δ194.74,174.60,162.81,158.99,157.38,153.45,137.53,130.04,128.91,128.22,127.09,126.96,124.05,123.35,117.63,115.02,113.60,101.12,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com