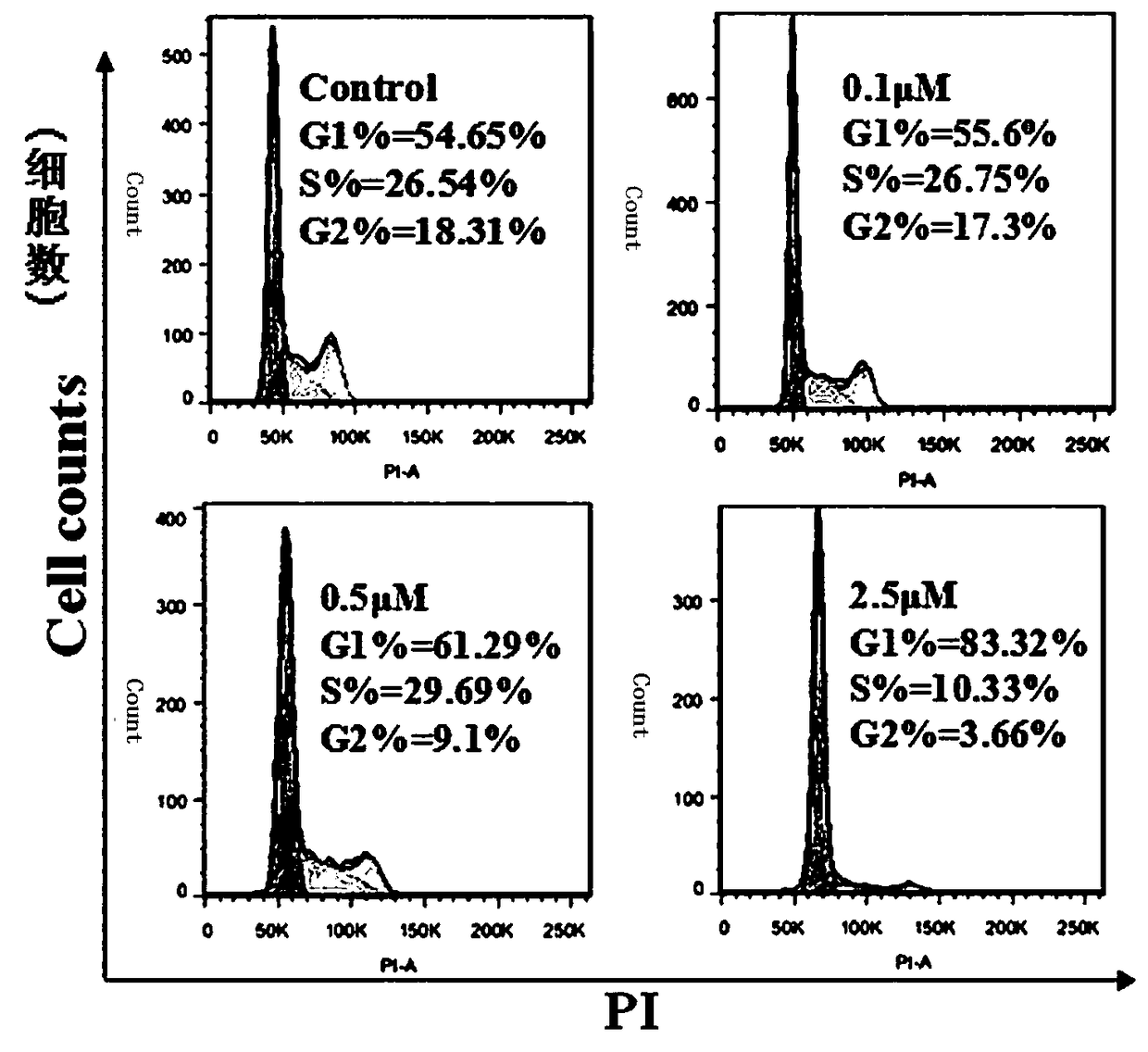
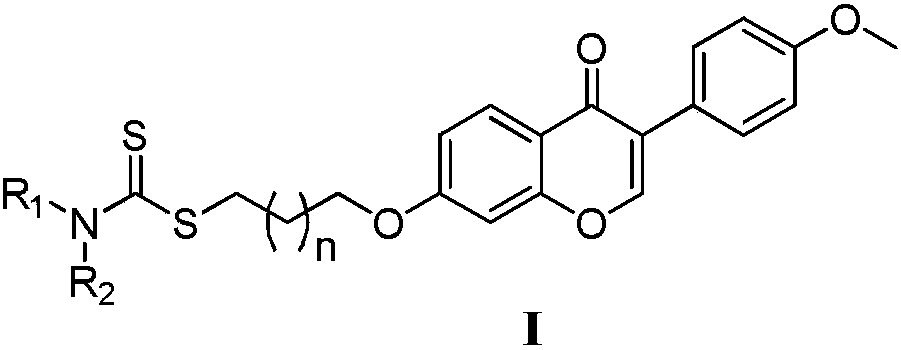
Formononetin derivatives containing carbadithiocarbamate, preparation method and application in antitumor drugs
A technology containing aminodithiocarbamate and formononetin, applied in antitumor drugs, drug combinations, organic chemistry, etc., can solve the problem of weak antitumor activity, improve antitumor activity, and solve the Weak tumor activity, the effect of broadening the research field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Preparation of general formula (1a~1d)
[0028] In 100ml tetrahydrofuran, react 10g formononetin, 6.2g potassium carbonate and 7g 1,2-dibromoethane or 7.5g 1,3-dibromopropane or 8g 1,4-dibromobutane at 80°C Heat and stir. After 6 hours of reaction, the system was concentrated to dryness, and 10 ml of ethanol was added for recrystallization to obtain compounds 1a to 1d.
Embodiment 2
[0029] The preparation of embodiment 2 general formula (I1~I9)
[0030] In 10ml of acetone, 1g of compound (1a~1d) was reacted with 600mg of substituted secondary amine, 300mg of carbon disulfide, and 440mg of sodium phosphate at room temperature. After the reaction was completed, suction filtration, the filtrate was concentrated, and 3ml of ethanol was recrystallized to obtain the general formula (I1~ I9) Compounds.
[0031] The general formula (I1~I9) structure is characterized as follows:
[0032] I-1: Yield 83%. White solid. Mp: 124~125℃. 1 H NMR (400MHz, DMSO) δ 8.42(s, 1H), 8.03(d, J=8.9Hz, 1H), 7.53(d, J=8.7Hz, 2H), 7.38–7.29(m, 4H), 7.29 –7.22(m,1H),7.17(d,J=2.2Hz,1H),7.09(dd,J=8.9,2.3Hz,1H),7.00(d,J=8.8Hz,2H),4.22(t, J=6.1Hz, 4H), 3.92(s, 2H), 3.79(s, 3H), 3.51(s, 2H), 3.42(t, J=7.2Hz, 2H), 2.45(s, 4H), 2.22– 2.09(m,2H). 13 C NMR(100MHz,DMSO)δ194.74,174.60,162.81,158.99,157.38,153.45,137.53,130.04,128.91,128.22,127.09,126.96,124.05,123.35,117.63,115.02,113.60,101...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com