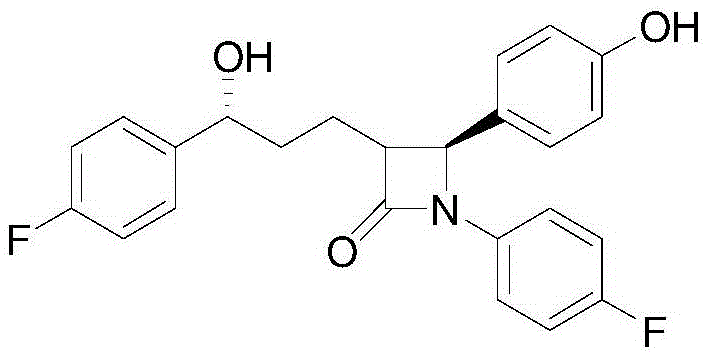
Ezetimibe dispersing tablet, and preparation method thereof
A technology of ezetimibe and dispersible tablets, applied in the field of medicine, can solve the problems of dissolution rate affecting bioavailability and curative effect, and achieve the effects of improving disintegration and dissolution, good taste and improving dispersibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] Preparation Example
[0021] The preparation method of ezetimibe dispersible tablet of the present invention comprises the following steps:
[0022] (1). Dissolve ezetimibe in 10 times the weight of ethanol according to Table 1. After absorbing the ezetimibe-containing ethanol solution with a filler, evaporate the ethanol;
[0023] (2). Mix the filler adsorbed with ezetimibe evenly with disintegrating agent, binder, lubricant and essence, and directly compress the tablet to obtain 100 tablets for each embodiment.
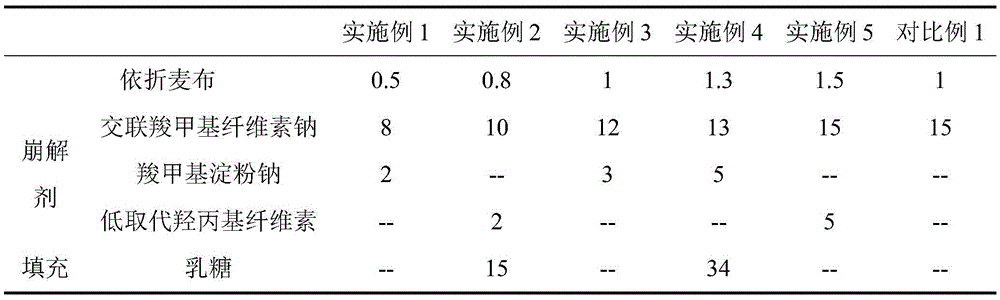
[0024]
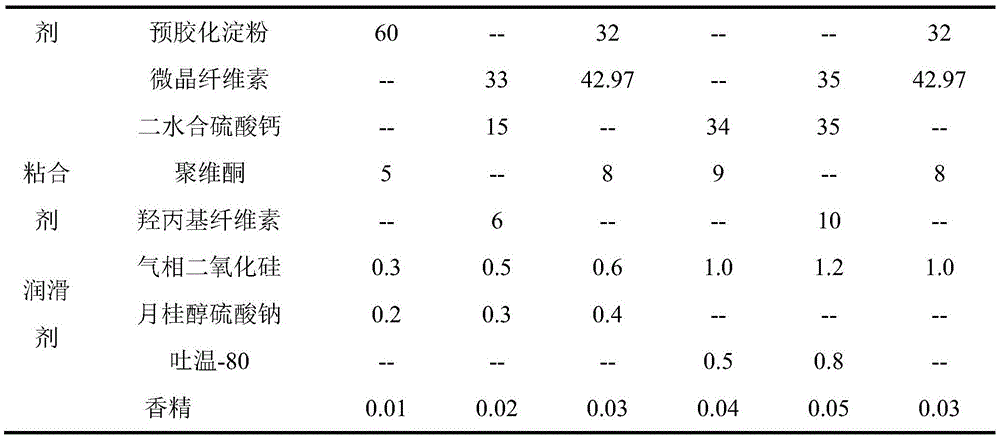
[0025]
[0026]
[0027] The filler can be one of the pharmaceutically acceptable fillers suitable for powder compression or used in combination. The binder may be one of the pharmaceutically acceptable binders suitable for powder compression.
[0028] Dispersion uniformity example
[0029] Carry out according to [dispersion uniformity] in Chinese Pharmacopoeia 2010 edition appendix IA tablet.
[0030] Take the tablets prepared in Exam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com